- Cet évènement est passé.
Conférence Dr Lorette Sicard

Dr Lorette Sicard
Laboratoire ITODYS, Université Paris Cité
Rational design of nanoalloys for catalytic dehydrogenation and transfer hydrogenation
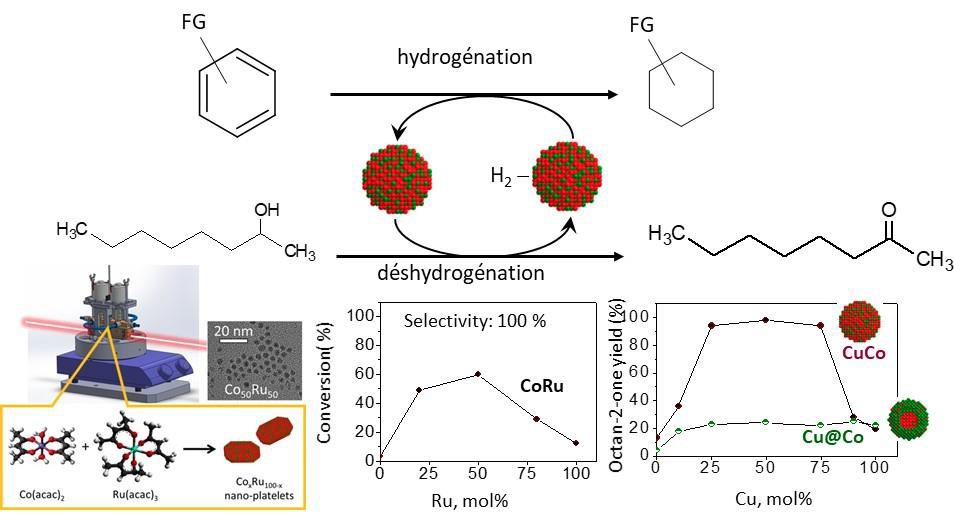
Nanoalloys have emerged as a key class of materials across various fields, particularly in catalysis, where they often display synergetic properties compared to monometallic nanoparticles (NPs) counterparts. Their catalytic activity can be finely tuned by optimizing both composition and nanostructure, opening avenues for performance improvements.
Among the soft chemistry routes for nanoalloy synthesis, the polyol process has proven especially attractive as the solvent simultaneously acts as a reducing and complexing agent and the synthesis temperature (180-300°C) is sufficiently high to promote good cristallinity. However, the morphology and structure of the resulting NPs are strongly influenced by numerous paramaters. An understanding of the mechanism of the reduction and formation mechanisms is therefore essential to rationalize synthesis strategies, particularly for bimetallic NPs. Spectroscopy techniques such as UV-visible and in situ X-ray absorption, complemented by other characterization methods, provide valuable insights into these mechanisms. This will be illustrated through the case study of CoRu nanoalloys. Furthermore, the improvements of the catalytic properties provided by alloying Co with other metals, Ru but also Cu, on the dehydrogenation of alcohols will be discussed as well as the issue of catalyst recyclability.
Finally, the first coupling of the alcohol dehydrogenation with the hydrogenation of a wide range of aryl molecules, achieved without the need for molecular hydrogen or other additives, will be presented. Such tranfer hydrogenations represent a green alternative to conventional hydrogenation strategies, avoiding the use of large amounts of solvents and of compressed, high pressure hydrogen gas, thus improving both safety and sustainability.