
- Cet évènement est passé.
Conférence Pr J.N.H. REEK

Pr J.N.H.Reek
Homogeneous and supramolecular catalysis, van ‘t Hoff institute for molecular sciences, University of Amsterdam, The Netherlands
E-Mail : j.n.h.reek@uva.nl
Self-assembled molecular cages for transition metal catalysis, nanoparticle formation and applications in living cells
The interface between supramolecular chemistry and transition metal catalysis has received surprisingly little attention in contrast to the individual disciplines. It provides, however, novel and elegant strategies that lead to new tools for the search of effective catalysts, and as such this has been an important research theme in our laboratories.[1] In this context we have intensively explored the use of well defined nanospheres[2,3] that form by self-assembly in transition metal catalysis. These nanospheres create catalysts (and substrates) at high local concentration, just like in enzymes, higher reaction rates are observed for several reactions that operate via binuclear mechanism. Also, they provide new tools to control catalytic events in complex media, showing substrate selective catalysis, effector controlled catalysis and catalysis with feed back loops. More recently we have translated the chemistry from the typical organic solvents to aqueous media and biorelevant conditions. This allows to use these nanostructure for new functions as gene delivery and nonnatural catalytic conversions in living cells.[4] In this lecture I will focus on 1) the application of cages in gold catalysis, 2) the controlled formation of caged nanoparticles, 3) and discuss the application of cages for gene delivery.
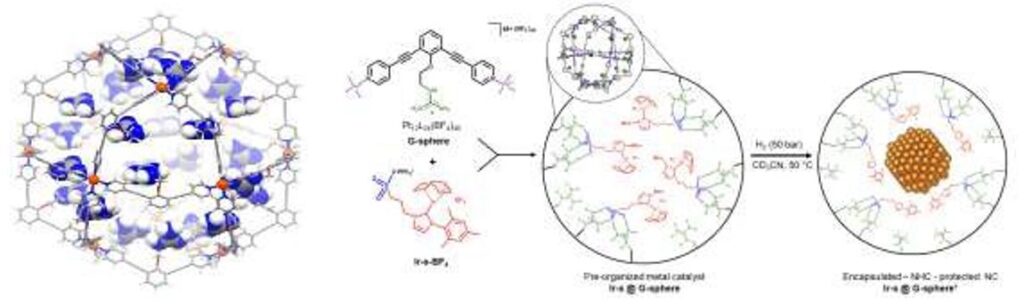
An example of a M12L24 nanosphere as scaffold to bind gold catalysts (left) and the strategy to form nanoparticles (right)
Références
- For reviews see: a) Reek et al, Nature Chemistry, 2010, 2, 615. b) Reek et al, Soc. Rev, 2015, 44, 433 – 448 c) Reek et al Chem. Soc. Rev. 2008, 37, 247. d) Reek et al., Acc. Chem. Res. 2018, 51, 2115. e) Reek et al ACS Catal. 2018, 8, 3469. f) Reek et al ChemAsianJ. 2021,16, 3851. g) Reek et al Chem. Sci., 2021, 12, 502. H) Reek et al Chem. Rev., 2022, 22, 12308 i) Reek et al Chem. Rev., 2023, 23, 52255
- Pioneering work on nanospheres: Fujita, a) Angew. Chem. Int. Ed. 2004, 43, 5621
b) Science 2010, 328, 1144 c) Commun. 2009, 13, 1638. d) J.P Stang et al. J. Am. Chem. Soc. 1999, 121, 10434. - For some of our work on nanospheres a) Reek et al., “Nature Chemistry, 2016 8, 225- 230; b) Reek et al Chem., Int. Ed., 2014, 52, 13380; c) Reek et al Angew. Chem., Int. Ed., 2018, 57, 11247; d) Reek et al Chem. Sci., 2019,10, 1316. e) Reek et al J. Am. Chem. Soc. 2020, 142 (19), 8837.f) Reek et al J. Am. Chem. Soc. 2022, 144, 15633
- A) Reek et al Chem 2023, 9, 1578, b) Reek et al Sci., 2023, 14, 6943
