- Cet évènement est passé.
Conférence Pr Ivan CASTILLO
jeudi 24 novembre 2022 de 11:00 - 12:00

Biologically-inspired Nickel and Iron proton reduction catalysts
Dr Ivan CASTILLO
Instituto de Química
Universidad Nacional Autónoma de México
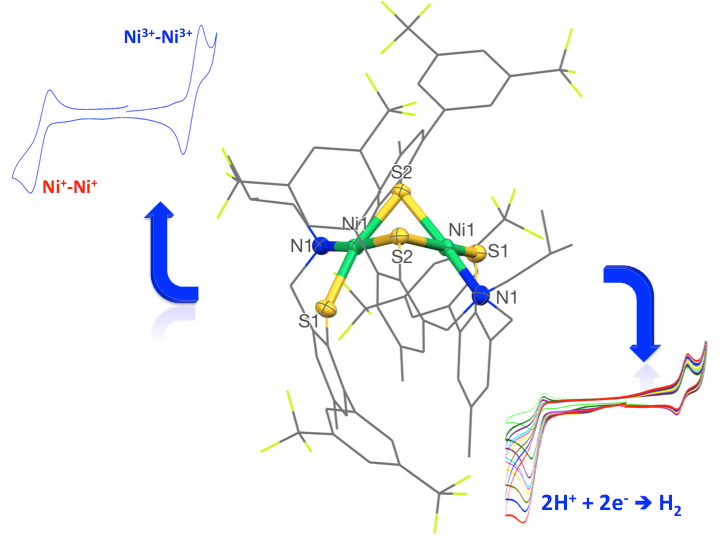
Metalloenzymes that carry out redox transformations employ sulfur rich active sites to coordinate transition metals. Among these, nickel-iron [NiFe] hydrogenases exploit cysteine residues to bridge both metal ions and favor their cooperative action in the generation of dihydrogen from protons. In this context, we have developed sulfur-rich ligands that may act as models of the active sites of such metalloenzymes and obtained the corresponding Nickel and Iron complexes. The hydrogenase properties of the nickel and iron complexes will be discussed.