
- Cet évènement est passé.
Conférence Pr Cameron JONES

Highly Activated Magnesium(I) Compounds:
Powerful Reagents for Small Molecule Activations
Pr Cameron JONES
School of Chemistry, Monash University, Melbourne, VIC, 3800, Australia
cameron.jones@monash.edu; Twitter: @Jones_Research
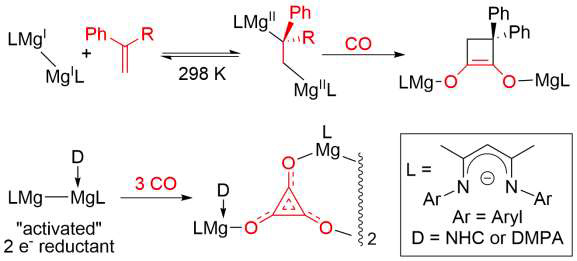
Since the synthesis of the first stable magnesium(I) compounds was achieved in 2007, the unique properties these species possess has lent them to use as versatile reducing agents in both organic and inorganic synthetic protocols[1] The products of such reactions are often inaccessible using more classical reducing agents. We have recently developed several classes of highly activated magnesium(I) and « masked » magnesium(I or 0) systems, and have shown these to be markedly more reactive than their established counterparts.[2-5] In this lecture it will be shown that these compounds are powerful reagents for the « transition metal-like » activation of catalytically relevant small molecules and inert arenes (e.g. CO, H2, C2H4, N2, C6H6) (Figure 1). In several cases, small molecule activations are redox reversible under mild conditions, which gives hope for the eventual incorporation of magnesium(I) compounds into catalytic cycles.
REFERENCES
[1] Jones, C. Nat. Rev. Chem., 2017, 1, 0059.
[2] Boutland, A. J.; Carroll, A.; Lamsfus, C. A.; Stasch, A.; Maron, L.; Jones, C., J. Am. Chem. Soc., 2017, 139, 18190.
[3] Yuvaraj, K.; Douair, I.; Paparo, A.; Maron, L.; Jones, C. J. Am. Chem. Soc., 2019, 141, 8764.
[4] Paparo, A.; Yuvaraj, K.; Matthews, A. J. R.; Douair, I.; Maron, L.; Jones, C., Angew. Chem. Int., 2021, 60, 630.
[5] Jones, D.D.L.; Douairm I.; Maron, L.; Jones, C., Angew. Chem. Int., 2021, 60, 7087.
