
- Cet évènement est passé.
Conférence Pr Med Abderrahmane K. SANHOURY

Synthesis, characterization and coordination chemistry of organophophorus chalcogenides
Med Abderrahmane Sanhoury 1,2
1Laboratory of Structural Organic Chemistry: Synthesis and Physicochemical Studies, Faculty of Sciences of Tunis, University of Tunis El-Manr, 2092, El Manar
I, Tunis, Tunisia,
2Materials Chemistry Research Unit, Faculty of Sciences and Techniques, University of Nouakchott Al Aasriya, Nouakchott, Mauritania
email: senhourry@yahoo.com
Among the large number of activities and properties reported for organophopshorus compounds (OPs), the following are particularly noteworthy: biological activities and potential agricultural applications, flame retardant properties, their use as antirust additives within lubricating oils and, notably, pharmaceuticals used for treating and controlling various diseases [1]. In addition to being used as prodrugs and intermediates in organic synthesis, OPs are widely used as ligands for transition metal catalyzed reactions due to their chemical versatility [2]. For instance, phosphine chalcogenides have been used extensively as transfer agents yielding a reactive source of chalcogenides [3]. In this presentation we will give an overview of the different approaches for the synthesis of various phosphoramides, phosphoamidates, phophine chalcogenides and corresponding diphosphoryl derivatives (1-9). Particularly in this context and as a continuation of our interest in organophosphorus chemistry [4-6], we will also describe their coordination chemistry towards both soft and hard metal ions. These products were fully characterized by multinuclear (1H, 13C and 31P) NMR, IR and in some cases by X-ray analyses. In addition, the effect of the nature of different phosphorus substituents on the reaction yield and complex stability will be compared and discussed.
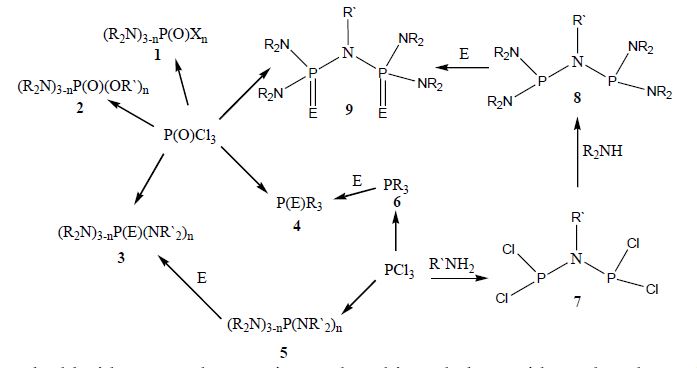
Keywords: Phosphoryl chloride, secondary amines, phosphine chalcogenides, phosphoramidates, metal complex, NMR.
References
[1] G. Keglevich, Organophosphorus Chemistry, vol. 42, Royal Society of Chemistry, 2013. pp. 49–80.
[2] R. Aznar, A. Grabulosa, A. Mannu, G. Muller, D. Sainz, V. Moreno, M. Font-Bardia, T. Calvet, J. Lorenzo, Organometallics 32 (2013) 2344.
[3] (a) D. Belletti, D. Cauzzi, C. Graiff, A. Minarelli, R. Pattacini, G. Predieri, A. Tiripicchio, J. Chem. Soc., Dalton Trans. 16 (2002) 3160; (b) P. Sekar, J.A. Ibers, Inorg. Chim. Acta 332 (2002) 123; (c) D. Belletti, C. Graiff, C. Massera, R. Pattacini, G. Predieri, A. Tiripicchio, Inorg. Chim. Acta 356 (2003) 187;
[4] M. A. Sanhoury, M. T. Ben Dhia, M. R. Khaddar J. Fluorine Chem. 146 (2013) 15.
[5] M. A. Sanhoury, T. Mbarek; A.M.Z. Slawin, M.T. Ben Dhia, M. R. Khaddar, J. D. Woollins Polyhedron 119 (2016) 106.
[6] F. Laribi, M.A.K. Sanhoury, H. Mechi, D. Merlet, I. Chehidi, Inorg. Chim Acta, 533 (2022) 120803.
