LCC
Nanoparticles and Catalysis
Diversity of Catalytic Systems and Reactions
Our research activities in nanocatalysis integrate the principles of green chemistry and sustainable development. They aim to develop innovative nanometric catalytic systems, precisely defined and exhibiting relevant catalytic properties in terms of activity and selectivity, while ensuring adequate stability to allow recycling without loss of performance.
Catalytic studies are conducted using species in solution or supported systems, under mild temperature and pressure conditions whenever possible. Among our objectives is the development of catalysts for hydrogenation, hydrogenolysis, dehydrogenation, hydroformylation, and other key reactions. Some systems are being developed for biomass valorization (e.g., lignin model molecules, furfural) into platform molecules or synthetic fuels. Collaborations with theoretical chemists allow us to better understand the reaction mechanisms involved.
Example for hydrogenolysis catalysis:
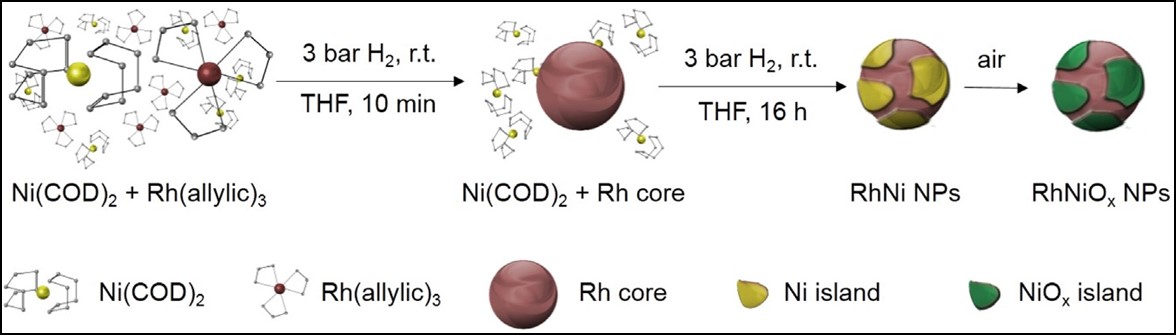
– To promote the selective hydrogenolysis of lignin model molecules, we synthesized rhodium nanoparticles decorated with nickel oxide islands on their surface. These islands partially block the rhodium surface, thus limiting hydrogenation in favor of hydrogenolysis.
(J. Mol. Catal. A: Chem. 2016, 422, 188-197; doi.org/10.1016/j.molcata.2016.01.014).
Examples for hydrogenation catalysis:
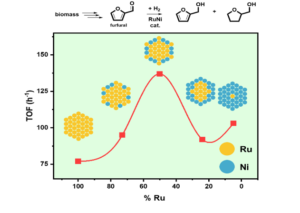
– The synthesis of RuNi nanocatalysts enabled the optimization of selective furfural hydrogenation. This work highlighted the benefits of Ru dilution through Ni association, the Ru/Ni synergy effects, and the influence of solvent nature on selectivity changes.
(Green Chemistry, 2021, 23, 8480-8500 ; doi.org/10.1039/D1GC02154K).
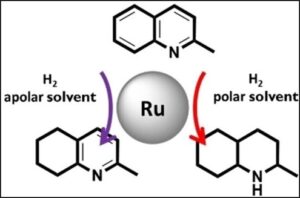
– Colloidal ruthenium nanoparticles appeared as selective catalysts for the hydrogenation of heterocycles at room temperature. Control over activity and chemoselectivity was achieved by varying both the reaction solvent and the stabilizing ligands. Hydrogenation of the carbocycle was obtained with up to 70% selectivity at full conversion.
(Chem. Eur. J. 2024, 30, e202302131, 1-9 ; doi.org/10.1002/chem.202302131).
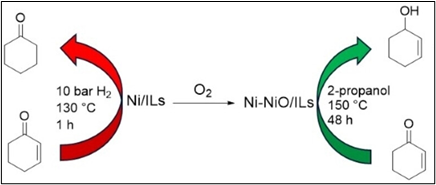
– Modulation of the oxidation state of nickel nanoparticles dispersed in functionalized ionic liquids allowed either chemoselective hydrogenation (Ni⁰) or transfer hydrogenation (Niᵒˣ) of α,β-unsaturated carbonyl compounds.
(ChemCatChem 2024, 16(4), e202301441/1-8; doi.org/10.1002/cctc.202301441).
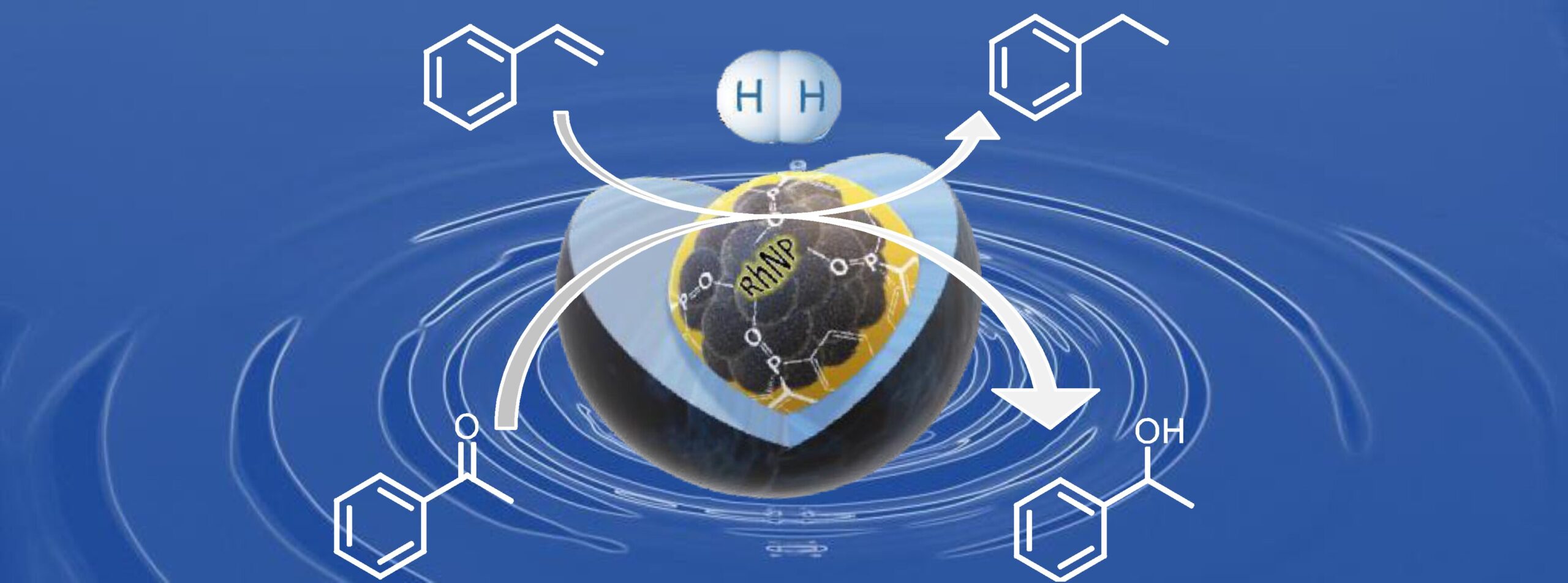
– The dispersion of rhodium nanoparticles within the micellar cores of cross-linked polymers functionalized with phosphine oxide ligands led to a catalytic system that is active, selective, and reusable for biphasic aqueous hydrogenation of styrene. These nanoreactors also proved applicable to the selective hydrogenation of other substrates, such as alkynes and carbonyl derivatives.
(ChemCatChem 2024, 16(15), e202400189/1-10; doi.org/10.1002/cctc.202400189).
These projects are conducted in collaboration with:
- In Toulouse: LPCNO (theoretical chemistry), CIRIMAT (XPS)
- In Spain: Autonomous University of Barcelona (UAB); Centro Mixto CSIC–Universidad de Sevilla
- In Denmark: Technical University of Denmark (DTU, Lyngby)
- In Singapore: National University of Singapore (NUS)
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




