LCC
Service overview
The mission of the NMR facility is to provide scientific and technical expertise in NMR spectroscopy and to provide users with open-access to a range of functional equipment. In particular, the facility carries out and interprets NMR spectra on specific problems. It can carry out detailed studies as part of scientific collaborations or service deliveries. It is also responsible for the maintenance of the spectrometers, training and assistance to users of open-access equipment.
The NMR service is open to researchers from outside the laboratory and from the private sector. The service is part of the “RMN Midi-Pyrénées” network.
Service members

COPPEL Yannick

BIJANI Christian

BONNET Antoine
Equipments
4 NMR spectrometers Bruker in open-access
Bruker NMR spectrometers Avance 300, Avance 400, 2 Avance III 400 (PDF file), 3 of which are equipped with autosamplers. Realisation of 1D experiments (1H, 13C, 31P, 11B, …), 2D experiments (COSY, HMQC, HMBC, NOESY…) and variable temperature experiments.
Note the possibility of carrying out experiments with double decoupling: 13C with 1H and 31P decoupling, HMQC 1H-13C with 31P decoupling…).


Bruker Avance III 400 Wide-Bore for solid-state NMR
Equipments:
- 4mm triple resonance probe MAS H-F/X/Y with insert 31P / 65Cu – 29
- 4mm double resonance probe MAS H/X low gamma (109Ag – 13C)
- 2mm double resonance probe MAS H-F/X (15N – 31P)
- 5mm double résonance probe MAS H-F/X (13C – 31P)
- 3mm double résonance probe MAS H/X (15N – 31P)
- 5mm goniometric probe H/X (15N – 31P)
- Variable temperature unit (BCUII + Nitrogen exchanger)

Bruker Avance NEO for liquid or solid-state NMR
Equipments:
- 5mm broadband inverse triple-resonance probe
1H, BB(31P-103Rh)/31P with Z field gradients
- 2mm double resonance CPMAS H/X high gamma (31P – 15N)
- 2mm double resonance CPMAS H/X low gamma (15N – 109Ag)
- Variable temperature unit (BCUII and nitrogen vaporization)
Services
Application form for high-resolution liquid NMR experiments (Analyses RMN liquide)
Application form for solid-state NMR experiments (Analyses RMN solide)
Rates: Contact us
The service applies a certified full-cost pricing system that allows the invoicing of services in research projects financed by public third parties (ANR, EU, etc.).
Application fields
NMR (Nuclear Magnetic Resonance) is a non-destructive chemical analysis technique commonly used to determine the molecular composition and purity of a sample.
It allows the identification of molecules, the determination of their structure and the study of some of their physical properties in solution or in the solid state (change of conformation, phase, solubility, size…).
This facility offers a wide range of services that can be integrated into your R&D projects in a wide variety of scientific fields (materials, organic chemistry, catalysis, natural products, etc.). Today, we carry out NMR measurements for more than twenty public or industrial laboratories.
The NMR facility can offer you solutions ranging from simple access to NMR spectrometers for routine controls to the complete handling of your samples by our engineers (preparation of samples for analysis, performance of measurements, interpretation of results, drafting of an analysis report, etc.). Any service will be subject to a detailed quote that we will draw up according to your needs.
Application examples:
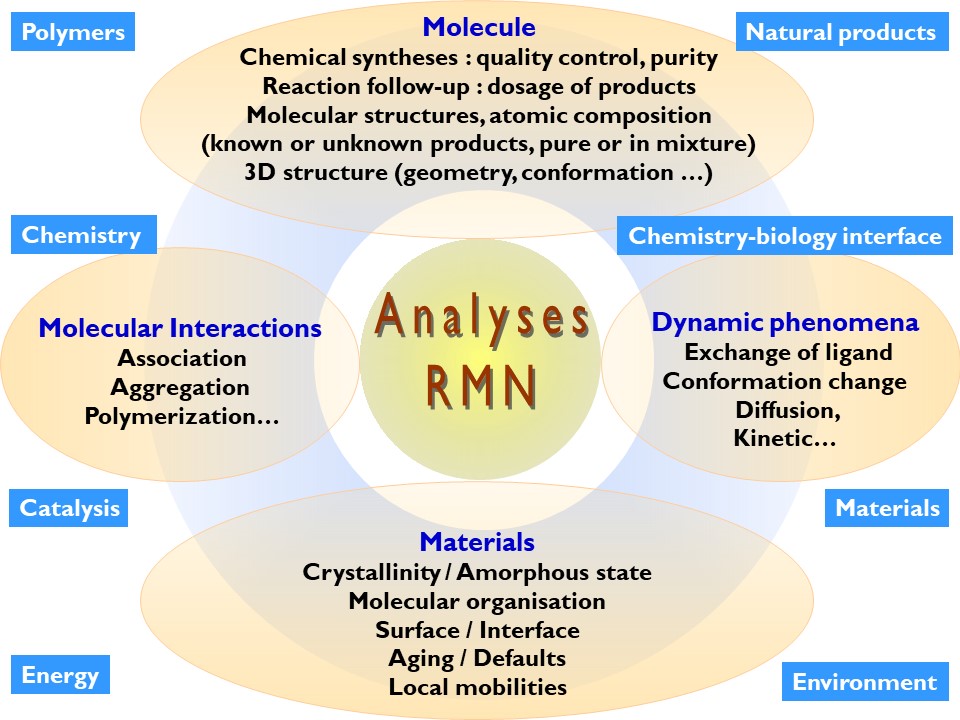
Monitoring of chemical syntheses
- Chemical composition of a reaction mixture
- Quality control of production
- Detection and characterisation of impurities
Determination of the molecular structure
- Study of the three-dimensional shape of molecules
- Identification of original molecules, bioactive molecules (antibiotics, antifungals…), natural products…
Study of materials or insoluble products
- Crystalline (polymorphism) or amorphous form
- Absorption of molecules on silicas, zeolites…
- Arrangement of molecules in the solid-state
- Natural products (wood, bone…)
- Gel, liquid crystals
- Aging/modification of materials
Study of dynamic phenomena
- Study of reaction mechanisms (kinetic and thermodynamic study, reaction intermediates)
- Isotope exchange
Study of molecular interactions
- Study of direct or reverse micelles, vesicles, liposomes
- Study of association phenomena, aggregation, polymerisation…
Polymer study
- Linear or branched form, in solution or solid state
- Structure of the units
- Polydispersity index
- Average molecular weight and size
Feasible experiments
Liquid -state samples:
– A lot of different nucleus can be studied: 1H, 2H, 7Li, 11B, 13C, 15N, 19F, 27Al, 29Si, 31P, 51V, 103Rh, 109Ag, 113Cd, 119Sn, 195Pt…
– 1D and 2D triple-resonance experiment can be run: 1H/31P/X (X {1H, 31P}, 31P {1H, X} and 1H {31P, X}). In addition triple resonance experiment 1H/13C/X et 1H/13C/15N/2H can be done on the spectrometers of « Midi-Pyrénées » NMR network.
– 1D multi-pulses experiments with gradient fields and selective pulses: DEPT, INEPT, T1 and T2 measurements, 1D TOCSY, 1D NOESY, 1D-HSQC, solvent suppression, 1D quantitative…
– 2D homo- and hetero-nuclear experiment: COSY, NOESY, TOCSY, ROESY, EXY, HMQC, HSQC, HMBC, ADEQUATE…
– 2D experiment with homonuclear 1H decoupling (pure-shift, psyche, ZS, HOBS…)
– DOSY experiment for self-diffusion coefficient measurement.
– Variable temperature (-100°C à +100°C).
Solid -state samples:
– A lot of different nucleus can be studied: 1H, 2H, 11B, 13C, 15N, 17O, 19F, 29Si, 31P, 33S, 35Cl, 51V, 77Se, 79Br, 107Ag, 113Cd,…
– Triple-resonance experiment can be run: 1H/31P/13C and 1H/19F/X
– 1D experiments: 1H/19F-X CP et CP quantitative, X{1H} HPDEC, INEPT, spectral edition, X CPMG et CP-CPMG, REDOR
– 2D experiments: 1H-19F/X HETCOR, HMQC, MQMAS, 2D DQMAS, NOESY-RFDR, 2D CRAMPS….
– Study of dynamic phenomena: relaxation time measurement (T1, T2, T1ρ), dipolar coupling and CSA measurement…
– Variable temperature (-60°C à +100°C)
Publications
2026
2025
How tailor-made copolymers can control the structure and properties of hybrid nanomaterials: the case of polyionic complexes
Peng L., Odnoroh M., Destarac M., Coppel Y., Delmas C., Benoit-Marquié F., Mingotaud C., Marty J.-D.
Nanoscale 2025, 17(8), 4636-4648.
10.1039/D4NR04332D – hal-04890110
Lanthanide(III)-dependent hydration of the methanol dehydrogenase cofactor, pyrroloquinoline quinone
Blanc C., Oriol L., Rajeshkumar T., Bijani C., Serpentini C.-L., Giraud N., Maron L., Hureau C., Mathieu E.
Inorg. Biochem. 2025, 270, 112924/1-8.
10.1016/j.jinorgbio.2025.112924 – hal-05085198
Luminescence quenching of pyrene-labelled fluorescent dendrons by surface anchoring of ruthenium nanoparticles
González-Gómez R., Vonlanthen M., Bijani C., Amiens C., Rivera E., Philippot K.
Dalton Trans. 2025, 54(19), 7851-7861.
10.1039/D5DT00192G – hal-05065873
Permethylated cyclodextrins with thiol groups as stabilizing agents for catalytic water-soluble platinum nanoparticles
Marchenko N., Jung S., Pham A., Creste G., Aygün M., Esvan J., Coppel Y., van Leeuwen P. W. N. M., Armspach D., Tricard S.
Eur. J. Inorg. Chem. 2025, 28(8), e202400776/1-8.
10.1002/ejic.202400776 – hal-05018587
Thiophosphate-based covalent Organic Framework (COF) or Porous Organic Polymer (POP)?
Menendez C., Coppel Y., Martin B., Caminade A.-M.
Macromol 2025, 5(1), 10/1-9.
10.3390/macromol5010010 – hal-05052505
When coordination chemistry meets soft matter: From hybrid nanoparticles to assemblies
Gauffre F., Coppel Y., Marty J.-D., Mingotaud C., Kahn M. L.
Coord. Chem. Rev. 2025, 543, 216893/1-20.
10.1016/j.ccr.2025.216893 – hal-05147629
Antibacterial and antibiofilm properties of two cyclic dipeptides produced by a new desert Streptomyces sp. HG-17 strain against multidrug-resistant pathogenic bacteria
Driche E.-H., Badji B., Bijani C., Belghit S., Pont F., Mathieu F., Zitouni A.
Int. Microbiol. 2025, 28, 241-255.
10.1007/s10123-024-00533-7 – hal-04804447
2024
Colloidal bimetallic RuNi particles and their behaviour in catalytic quinoline hydrogenation
Cardona-Farreny M., Ishikawa H., Odufejo Ogoe A. O., Mallet-Ladeira S., Coppel Y., Lecante P., Esvan J., Philippot K., Axet M. R.
ChemPlusChem 2024, e202400516/1-10.
https://doi.org/10.1002/cplu.202400516
https://hal.science/hal-04703442
Antibacterial and antibiofilm properties of two cyclic dipeptides produced by a new desert Streptomyces sp. HG-17 strain against multidrug-resistant pathogenic bacteria
Driche E.-H., Badji B., Bijani C., Belghit S., Pont F., Mathieu F., Zitouni A.
International Microbiology 2024,
https://doi.org/10.1007/s10123-024-00533-7
https://hal.science/hal-04804447
New hybrid adsorbent based on APTES functionalized zeolite W for lead and cadmium ions removal: Experimental and theoretical studies
Abdellaoui Y., El Ibrahimi B., Ahrouch M., Kassab Z., El Kaim Billah R., Coppel Y., López-Maldonado E. A., Abou Oualid H., Díaz de León J. N., Leiviskä T., Giácoman-Vallejos G., Gamero-Melo P.
Chemical Engineering Journal 2024, 499, 156056/1-15.
https://doi.org/10.1016/j.cej.2024.156056
https://hal.science/hal-04804325
Seven-coordinate group 6 metal hydrides obtained by H2 activation at B(C6F5)3 adducts of N2 complexes: frustrated Lewis pair-type reactivity of the B–N linkage
Boegli M.-C., Coffinet A., Bijani C., Simonneau A.
Chemistry – An Asian Journal 2024, 19(12), e202400451/1-6.
https://doi.org/10.1002/asia.202400451
https://hal.science/hal-04633325
Unraveling the facet-dependent surface chemistry at molecular scale: Photoassisted oxidation of inp nanocrystals
Cho E., Kim M., Ouyang L., Kim H., Bonifas G., Coppel Y., Nayral C., Delpech F., Jeong S.-J.
Journal of the American Chemical Society 2024, 146(46), 31691-31701.
https://doi.org/10.1021/jacs.4c10231
https://hal.science/hal-04777854
Synthesis of TiO2/SBA-15 nanocomposites by hydrolysis of organometallic Ti precursors for photocatalytic NO abatement
El Atti O., Hot J., Fajerwerg K., Lorber C., Lebeau B., Ryzhikov A., Kahn M., Collière V., Coppel Y., Ratel-Ramond N., Ménini P., Fau P.
Inorganics 2024, 12(7), 183/1-19.
https://doi.org/10.3390/inorganics12070183
https://hal.science/hal-04659095
Unprecedented linear products by a mechanochemically activated Biginelli reaction using lawsone
Koumpoura C. L., Vendier L., Bijani C., Robert A., Carbonnière P., Sotiropoulos J.-M., Baltas M.
RSC Mechanochemistry 2024, 1(2), 167-175.
http://dx.doi.org/10.1039/D3MR00032J
https://hal.science/hal-04550806
Towards chitosan-amorphous calcium phosphate nanocomposite: Co-precipitation induced by spray drying
Le Grill S., Coppel Y., Soulie J., Bertrand G., Rey C., Brouillet F.
Next Materials 2024, 4, 100095/1-8.
https://doi.org/10.1016/j.nxmate.2023.100095
https://hal.science/hal-04416545
Consolidation of spray-dried amorphous calcium phosphate by ultrafast compression: Chemical and structural overview
Le Grill S., Drouet C., Marsan O., Coppel Y., Mazel V., Barthelemy M.-C., Brouillet F.
Nanomaterials 2024, 14(2), 152/1-14.
https://doi.org/10.3390/nano14020152
https://hal.science/hal-04472495
Hafnium coordination vitrimer based on carboxylate exchange: synthesis, properties, and mechanistic investigations on the [Hf6O4(OH)4(O2CMe)12]2 model compound
Murali M., Berne D., Daran J.-C., Bijani C., Manoury E., Leclerc E., Caillol S., ladmiral V., Joly-Duhamel C., Poli R.
European Journal of Inorganic Chemistry 2024, 27(8), e202300672/1-11.
https://doi.org/10.1002/ejic.202300672
https://hal.science/hal-04427867
Characterization by NMR spectroscopy of the SEI layer formed on Ti3C2 MXene materials prepared with various terminations
Sunny S., Coppel Y., Taberna P. L., Simon P.
Journal of The Electrochemical Society 2024, 171(3), 030512/1-7.
https://dx.doi.org/10.1149/1945-7111/ad2d1a
https://hal.science/hal-04786279
Spray-dried ternary bioactive glass microspheres: Direct and indirect structural effects of copper-doping on acellular degradation behavior
Vecchio G., Darcos V., Le Grill S., Brouillet F., Coppel Y., Duttine M., Pugliara A., Combes C., Soulié J.
Acta Biomaterialia 2024, 181, 453-468.
https://doi.org/10.1016/j.actbio.2024.05.003
https://hal.science/hal-04607086
Room temperature spin crossover properties in a series of mixed-anion Fe(NH2trz)3(BF4)2−x(SiF6)x/2 complexes
Yang X., Enriquez-Cabrera A., Jacob K., Coppel Y., Salmon L., Bousseksou A.
Dalton Transactions 2024, 53(15), 6830-6838.
http://dx.doi.org/10.1039/D4DT00267A
https://hal.science/hal-04529072
From stilbenes to carbo-stilbenes: An encouraging prospect
Zhu C., Saquet A., Maraval V., Bijani C., Cui X., Poater A., Chauvin R.
Chemistry – A European Journal 2024, 30(26), e202400451/1-7.
https://doi.org/10.1002/chem.202400451
https://hal.science/hal-04532883
2023
An experimental and computational investigation rules out direct nucleophilic addition on the N2 ligand in manganese dinitrogen complex [Cp(CO)2Mn(N2)]
Le Dé Q., Bouammali A., Bijani C., Vendier L., Del Rosal I., Valyaev D. A., Dinoi C., Simonneau A.
Angewandte Chemie, International Edition 2023, 62(40), e202305235/1-10.
https://doi.org/10.1002/anie.202305235
https://hal.science/hal-04159061
Spin crossover in mixed-anion Fe(NH2trz)3(BF4)(SiF6)0.5 crystalline rod-shaped particles: the strength of the solid–liquid post synthetic modification
Yang X., Enriquez-Cabrera A., Toha D., Coppel Y., Salmon L., Bousseksou A.
Dalton Transactions 2023, 52(30), 10828-10834.
http://dx.doi.org/10.1039/D3DT02003G
https://hal.science/hal-04174161
Gelation mechanism revealed in organometallic gels: Prevalence of van der Waals interactions on oligomerization by coordination chemistry
Wang Y., Coppel Y., Fitremann J., Massou S., Mingotaud C., Kahn M. L.
ChemPhysChem 2023, 24(14), e202300077/1-7.
https://doi.org/10.1002/cphc.202300077
https://hal.science/hal-04123076
N-heterocyclic carbene-iridium complexes as photosensitizers for in vitro photodynamic therapy to trigger non-apoptotic cell death in cancer cells
Wang X., Zhang C., Madji R., Voros C., Mazères S., Bijani C., Deraeve C., Cuvillier O., Gornitzka H., Maddelein M.-L., Hemmert C.
Molecules 2023, 28(2), 691/1-29.
https://doi.org/10.3390/molecules28020691
https://hal.science/hal-03870710
Acetate exchange mechanism on a Zr12 oxo hydroxo cluster: Relevance for reshaping zr-carboxylate coordination adaptable networks
Murali M., Bijani C., Daran J.-C., Manoury E., Poli R.
Chemical Science 2023, 14(30), 8152-8163.
http://dx.doi.org/10.1039/D3SC02204H
https://hal.science/hal-04155107
Mechanochemical studies on coupling of hydrazines and hydrazine amides with phenolic and furanyl aldehydes-hydrazones with antileishmanial and antibacterial activities
Kapusterynska A., Bijani C., Paliwoda D., Vendier L., Bourdon V., Imbert N., Cojean S., Loiseau P. M., Recchia D., Scoffone V. C., Degiacomi G., Akhir A., Saxena D., Chopra S., Lubenets V., Baltas M.
Molecules 2023, 28(13), 5284/1-26.
https://doi.org/10.3390/molecules28135284
https://hal.science/hal-04157067
Effect of oxygen poisoning on the bidirectional hydrogen electrocatalysis in TaS2 nanosheets
Ghorbani Shiraz H., Ullah Khan Z., Péré D., Liu X., Coppel Y., Fahlman M., Vagin M., Chmielowski R., Kahn M. L., Berggren M., Crispin X.
The Journal of Physical Chemistry C 2023, 127(12), 5825-5832.
https://doi.org/10.1021/acs.jpcc.3c00825
https://hal.science/hal-04342072
Synthesis and coordination of a bisphosphine-[NHC-borane] compound: A ligand framework for bimetallic structure featuring a boron-bridging moiety
Camy A., Vendier L., Bijani C., Fernández I., Bontemps S.
Inorganic Chemistry 2023, 62(23), 9035-9043.
https://doi.org/10.1021/acs.inorgchem.3c00785
https://hal.science/hal-04169429
Ultra-high-field 67Zn and 33S NMR studies coupled with DFT calculations reveal the structure of ZnS nanoplatelets prepared by an organometallic approach
Bellan E. V., Maleki F., Jakoobi M., Fau P., Fajerwerg K., Lagarde D., Balocchi A., Lecante P., Trébosc J., Xu Y., Gan Z., Pautrot-d’Alençon L., Le Mercier T., Nagashima H., Pacchioni G., Lafon O., Coppel Y., Kahn M. L.
The Journal of Physical Chemistry C 2023, 127(36), 17809-17819.
https://doi.org/10.1021/acs.jpcc.3c02754
https://hal.science/hal-04271597
Functionalization of graphene oxide surfaces with phosphorus dendrimer and dendron
Alami O., Laurent R., Tassé M., Coppel Y., Collière V., Bignon J., Majoral J.-P., El Kazzouli S., El Brahmi N., Caminade A.-M.
FlatChem 2023, 42, 100564/1-12.
https://doi.org/10.1016/j.flatc.2023.100564
https://hal.science/hal-04268533
“Click” chemistry for the functionalization of graphene oxide with phosphorus dendrons: Synthesis, characterization and preliminary biological properties
Alami O., Laurent R., Tassé M., Coppel Y., Bignon J., El Kazzouli S., Majoral J.-P., El Brahmi N., Caminade A.-M.
Chemistry – A European Journal 2023, 29(66), e202302198/1-10.
https://doi.org/10.1002/chem.202302198
https://hal.science/hal-04269848
2022
Dentin interaction with universal adhesive containing isopropanol solvent studied by solid-state NMR spectroscopy
Coppel Y., Nasr K., Prigent Y., Grégoire G.
Dental Materials 2022, 38(1), 7-18.
https://doi.org/10.1016/j.dental.2021.10.001
https://hal.archives-ouvertes.fr/hal-03669306
A new Saharan strain of streptomyces sp. GSB-11 produces maculosin and N-acetyltyramine active against multidrug-resistant pathogenic bacteria
Driche E. H., Badji B., Bijani C., Belghit S., Pont F., Mathieu F., Zitouni A.
Current Microbiology 2022, 79(10), 298/1-10.
https://doi.org/10.1007/s00284-022-02994-3
https://hal.archives-ouvertes.fr/hal-03789449
Hybrid polymeric micelles stabilized by gallium ions: Structural investigation
Gineste S., Lonetti B., Yon M., Giermanska J., Di Cola E., Sztucki M., Coppel Y., Mingotaud A.-F., Chapel J.-P., Marty J.-D., Mingotaud C.
Journal of Colloid and Interface Science 2022, 609, 698-706.
https://doi.org/10.1016/j.jcis.2021.11.077
https://hal.archives-ouvertes.fr/hal-03630399
Fac-to-mer isomerization triggers hydride transfer from Mn(I) complex fac-[(dppm)Mn(CO)3H]
Osipova E. S., Gulyaeva E. S., Kireev N. V., Kovalenko S. A., Bijani C., Canac Y., Valyaev D. A., Filippov O. A., Belkova N. V., Shubina E. S.
Chemical Communications 2022, 58(32), 5017-5020.
http://dx.doi.org/10.1039/D2CC00999D
https://hal.archives-ouvertes.fr/hal-03652224
Nano-structuration of WO3 nanoleaves by localized hydrolysis of an organometallic Zn precursor: Application to photocatalytic NO2 abatement
Castello Lux K., Fajerwerg K., Hot J., Ringot E., Bertron A., Collière V., Kahn M. L., Loridant S., Coppel Y., Fau P.
Nanomaterials 2022, 12(24), 4360/1-20.
https://doi.org/10.3390/nano12244360
https://hal.science/hal-03965121
3R-TaS2 as an intercalation-dependent electrified interface for hydrogen reduction and oxidation reactions
Ghorbani Shiraz H., Ullah Khan Z., Péré D., Liu X., Coppel Y., Fahlman M., Berggren M., Chmielowski R., Kahn M. L., Vagin M., Crispin X.
The Journal of Physical Chemistry C 2022, 126(40), 17056-17065.
https://doi.org/10.1021/acs.jpcc.2c04290
https://hal.archives-ouvertes.fr/hal-03877013
Design of anti-infectious agents from lawsone in a three-component reaction with aldehydes and isocyanides
Koumpoura C. L., Nguyen M., Bijani C., Vendier L., Salina E. G., Buroni S., Degiacomi G., Cojean S., Loiseau P. M., Benoit-Vical F., García-Sosa A. T., Robert A., Baltas M.
ACS Omega 2022, 7(40), 35635-35655.
https://doi.org/10.1021/acsomega.2c03421
https://hal.archives-ouvertes.fr/hal-03818601
Synthesis and antimalarial activities of new hybrid Atokel molecules
Li Y., Loureiro A., Nguyen M., Laurent M., Bijani C., Benoit-Vical F., Robert A., Liu Y., Meunier B.
ChemistryOpen 2022, 11(5), e202200064/1-20.
https://doi.org/10.1002/open.202200064
https://hal.archives-ouvertes.fr/hal-03681793
2021
Characterization of hydrogenated dentin components by advanced 1H solid-state NMR experiments
Coppel Y., Prigent Y., Grégoire G.
Acta Biomaterialia 2021, 120, 156-166.
https://doi.org/10.1016/j.actbio.2020.08.022
https://hal.archives-ouvertes.fr/hal-03202730v1
Spray-drying-derived amorphous calcium phosphate: a multi-scale characterization
Le Grill S., Soulie J., Coppel Y., Roblin P., Lecante P., Marsan O., Charvillat C., Bertrand G., Rey C., Brouillet F.
Journal of Materials Science 2021, 56(2), 1189-1202.
https://doi.org/10.1007/s10853-020-05396-7
https://hal.archives-ouvertes.fr/hal-03141573
A combined theoretical/experimental study highlighting the formation of carbides on Ru nanoparticles during CO hydrogenation
Moraru I.-T., Martínez-Prieto L. M., Coppel Y., Chaudret B., Cusinato L., Del Rosal I., Poteau R.
Nanoscale 2021, 13(14), 6902-6915.
http://dx.doi.org/10.1039/D0NR08735A
https://hal.archives-ouvertes.fr/hal-03202748v1
Oxidation-promoted synthesis of ferrocenyl planar chiral rhodium(III) complexes for C–H functionalization catalysis
Cabanes J., Odnoroh M., Duhayon C., Bijani C., Sournia-Saquet A., Polia R., Labande A.
Mendeleev Communications 2021, 31(5), 620-623.
https://doi.org/10.1016/j.mencom.2021.09.010
https://hal.archives-ouvertes.fr/hal-03412564
Amphiphilic polymeric nanoreactors containing Rh(i)–NHC complexes for the aqueous biphasic hydrogenation of alkenes
Salomon Sambou S., Hromov R., Ruzhylo I., Wang H., Allandrieu A., Sabatier C., Coppel Y., Daran J.-C., Gayet F., Labande A., Manoury E., Poli R.
Catalysis Science & Technology 2021, 11, 6811-6824
http://dx.doi.org/10.1039/D1CY00554E
https://hal.archives-ouvertes.fr/hal-0317189
Synthesis and reactivity of phosphine borohydride compounds
Ayyappan R., Coppel Y., Vendier L., Ghosh S., Sabo-Etienne S., Bontemps S.
Chemical Communications 2021, 57(3), 375-378.
http://dx.doi.org/10.1039/D0CC07072F
https://hal.archives-ouvertes.fr/hal-03149993
Anisotropic growth of ZnO nanoparticles driven by the structure of amine surfactants: the role of surface dynamics in nanocrystal growth
Wang Y., Coppel Y., Lepetit C., Marty J.-D., Mingotaud C., Kahn M. L.
Nanoscale Advances 2021, 3(21), 6088-6099.
http://dx.doi.org/10.1039/D1NA00566A
https://hal.archives-ouvertes.fr/hal-03353368
Nanocatalysts for high selectivity enyne cyclization: Oxidative surface reorganization of gold sub-2-nm nanoparticle networks
Nasrallah H. O., Min Y., Lerayer E., Nguyen T.-A., Poinsot D., Roger J., Brandès S., Heintz O., Roblin P., Jolibois F., Poteau R., Coppel Y., Kahn M. L., Gerber I. C., Axet M. R., Serp P., Hierso J.-C.
JACS Au 2021, 1(2), 187-200.
https://doi.org/10.1021/jacsau.0c00062
https://hal.archives-ouvertes.fr/hal-03129914
Trainings
Within the framework of the « Midi-Pyrénées » NMR network, we offer two NMR training courses through the « CNRS Formation Entreprise » organization (in French):
NMR analysis: acquisition, processing and interpretation (NMR analysis)
NMR for chemistry and biochemistry: advanced (advanced)
Each year, the NMR facility provides NMR training to the doctoral school of material sciences at the Paul Sabatier University of Toulouse (in English).
LCC
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France