LCC
Overview
The ALAMBIC team is interested in the roles of metal ions in life. Its main research line focuses on the role of copper and zinc ions in Alzheimer’s disease and in other processes involving amyloid-forming peptides.
Three axes are developed:
(i) A in-depth understanding at the molecular level of the interactions between metal ions and peptides;
(ii) The identification of new chelation-based concepts targeting copper ions bound to peptides, in order to minimize their harmful effects;
(iii) The design of new probes for the detection of amyloids and amyloids formation.
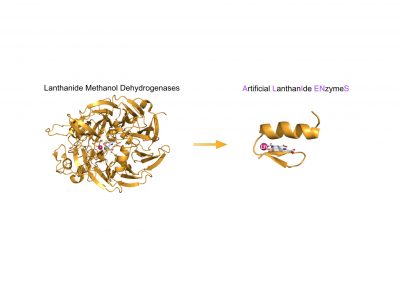
An emerging thematic in the team focuses on the design of artificial lanthanide enzymes.
Members of the team

HUREAU Christelle

BLANC Camille

BRISON Adèle

CAILLAT Antoine

DE CREMOUX Lucie

DROMMI Marielle

ESMIEU Charlène

FALCONE Enrico

FONDER Alexandre

GARNIER Maritie

LANTIGNER Lielou

LEFEVRE Margot

MARINHO-BARBOSA Barbara

MASSOT Mélanie

MATHIEU Emilie

PATRA Usharani

PRATVIEL Geneviève

RULMONT Clément

SCHMITT David
No Results Found
Research topics
Molecular basis of metal ions – amyloids interaction
Principal investigator: C. Hureau
Development of new therapeutic approaches
Principal investigator: C. Esmieu/C. Hureau
Artificial Lanthanide Enzymes
Principal investigator: E. Mathieu
Team news
CNRS Silver Medal for Christelle Hureau
This recognition highlights her remarkable career and outstanding contributions to coordination chemistry.
Alambic seminar day
3 plenary conferences at 11:00 AM, 2:00 PM and 3:40 PM.
2023 Mid-term PhD sessions at LCC
Congratulations to the students for their nice presentations !
Publications
2024
Star-like polypeptides as simplified analogues of horseradish peroxidase (HRP) metalloenzymes
Tronnet A., Salas-Ambrosio P., Roman R., Bravo-Anaya L. M., Ayala M., Bonduelle C.
Macromolecular Bioscience 2024, 2400155/1-10.
https://doi.org/10.1002/mabi.202400155
https://hal.science/hal-04692147
Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
Paoli-Lombardo R., Primas N., Hutter S., Castera-Ducros C., Jacquet I., Verhaeghe P., Azas N., Rathelot P., Vanelle P.
Heterocyclic Communications 2024, 30(1), 20220176/1-11.
https://doi.org/10.1515/hc-2022-0176
https://hal.science/hal-04675593
Bioinorganic chemistry of copper: from biochemistry to pharmacology
Da Costa Ferreira A. M., Hureau C., Facchin G.
Inorganics 2024, 12(4), 97/1-5.
https://doi.org/10.3390/inorganics12040097
https://hal.science/hal-04669017
Restoring cellular copper homeostasis in Alzheimer disease: a novel peptide shuttle is internalized by an ATP-dependent endocytosis pathway involving Rab5- and Rab14-endosomes
Okafor M., Champomier O., Raibaut L., Ozkan S., El Kholti N., Ory S., Chasserot-Golaz S., Gasman S., Hureau C., Faller P., Vitale N.
Frontiers in Molecular Biosciences 2024, 11, 1355963/1-15.
https://doi.org/10.3389/fmolb.2024.1355963
https://hal.science/hal-04668999
Rationally designed Cu(I) ligand to prevent CuAβ-generated ROS production in the Alzheimer’s disease context
Rulmont C., Stigliani J.-L., Hureau C., Esmieu C.
Inorganic Chemistry 2024, 63(5), 2340-2351.
https://doi.org/10.1021/acs.inorgchem.3c02693
https://hal.science/hal-04669002
Synthesis of nitrostyrylthiazolidine-2,4-dione derivatives displaying antileishmanial potential
Khoumeri O., Hutter S., Primas N., Castera-Ducros C., Carvalho S., Wyllie S., Efrit M. L., Fayolle D., Since M., Vanelle P., Verhaeghe P., Azas N., El-Kashef H.
Pharmaceuticals 2024, 17(7), 878/1-23.
https://doi.org/10.3390/ph17070878
https://hal.science/hal-04635068
Synthesis of new 5- or 7-substituted 3-nitroimidazo[1,2-a]pyridine derivatives using SNAr and palladium-catalyzed reactions to explore antiparasitic structure–activity relationships
Paoli-Lombardo R., Primas N., Bourgeade-Delmas S., Sournia-Saquet A., Castera-Ducros C., Jacquet I., Verhaeghe P., Rathelot P., Vanelle P.
Synthesis 2024, 56(8), 1297-1308.
http://doi.org/10.1055/a-2232-8113
https://hal.science/hal-04503326
Target fishing reveals PfPYK-1 and PfRab6 as potential targets of an antiplasmodial 4-anilino-2-trichloromethylquinazoline hit compound
Kieffer C., Primas N., Hutter S., Merckx A., Reininger L., Bach S., Ruchaud S., Gaillard F., Laget M., Amrane D., Hervé L., Castera-Ducros C., Renault J., Dumètre A., Rault S., Doerig C., Rathelot P., Vanelle P., Azas N., Verhaeghe P.
Bioorganic & Medicinal Chemistry 2024, 102, 117654/1-11.
https://doi.org/10.1016/j.bmc.2024.117654
https://hal.science/hal-04503278
2023
Effect of metal environment and immobilization on the catalytic activity of a Cu superoxide dismutase mimic
Richezzi M., Ferreyra J., Signorella S., Palopoli C., Terrestre G., Pellegri N., Hureau C., Signorella S. R.
Inorganics 2023, 11(11), 425/1-19.
https://doi.org/10.3390/inorganics11110425
https://hal.science/hal-04301970
Synthesis, characterization, and biological properties of the copper(II) complexes with novel ligand: N-[4-({2-[1-(pyridin-2-yl)ethylidene]hydrazinecarbothioyl}amino)phenyl]acetamide
Rusnac R., Garbuz O., Chumakov Y., Tsapkov V., Hureau C., Istrati D., Gulea A.
Inorganics 2023, 11(10), 408/1-17.
https://doi.org/10.3390/inorganics11100408
https://hal.science/hal-04302011
Reduced Schiff-base derivatives to stop reactive oxygen species production by the Cu(Aβ) species: a structure–activity relationship
Lefèvre M., Lantigner L., Andolfo L., Vanucci-Bacqué C., Benoist E., Esmieu C., Bedos-Belval F., Hureau C.
Comptes Rendus Chimie 2023, 26(S3), 1-11.
https://doi.org/10.5802/crchim.255
https://hal.science/hal-04299723
Novel thienopyrimidones targeting hepatic and erythrocytic stages of Plasmodium parasites with increased microsomal stability
Lagardère P., Mustière R., Amanzougaghene N., Hutter S., Casanova M., Franetich J.-F., Tajeri S., Malzert-Fréon A., Corvaisier S., Since M., Azas N., Vanelle P., Verhaeghe P., Primas N., Mazier D., Masurier N., Lisowski V.
European Journal of Medicinal Chemistry 2023, 261, 115873/1-12.
https://doi.org/10.1016/j.ejmech.2023.115873
https://hal.science/hal-04503282
LPMO-like activity of bioinspired copper complexes: from model substrate to extended polysaccharides
Leblay R., Delgadillo-Ruiz R., Decroos C., Hureau C., Réglier M., Castillo Pérez I., Faure B., Simaan A. J.
ChemCatChem 2023, 15(23), e202300933/1-9.
https://doi.org/10.1002/cctc.202300933
https://hal.science/hal-04231361
Interfacial behaviour of oligodeoxynucleotides prone to G-quadruplex formation on negatively charged electrode surface monitored by electrochemical probes
Dobrovodsky D., Danhel A., Mothes-Martin E., Pratviel G., Renciuk D., Mergny J.-L., Fojta M.
Electrochimica Acta 2023, 442, 141878/1-8.
https://doi.org/10.1016/j.electacta.2023.141878
https://hal.science/hal-04232807
Redox processes in Cu-binding proteins: the “in-between” states in intrinsically disordered peptides
Falcone E., Hureau C.
Chemical Society Reviews 2023, 52(19), 6595-6600.
http://dx.doi.org/10.1039/D3CS00443K
https://hal.science/hal-04231237
Can the level of copper in the hippocampus witness type-II diabetes versus Alzheimer’s disease?
Hureau C.
eBioMedicine 2023, 87, 104403/1-2.
https://doi.org/10.1016/j.ebiom.2022.104403
https://hal.science/hal-04231411
The critical role of ligand flexibility on the activity of free and immobilized Mn superoxide dismutase mimics
Richezzi M., Signorella S., Palopoli C., Pellegri N., Hureau C., Signorella S. R.
Inorganics 2023, 11(9), 359/1-22.
https://doi.org/10.3390/inorganics11090359
https://hal.science/hal-04231291
Biomimetic catalysis of nitrite reductase enzyme using copper complexes in chemical and electrochemical reduction of nitrite
Ferreira M. P., Castro C. B., Honorato J., He S.-Y., Gonçalves Guimarães Júnior W., Esmieu C., Castellano E. E., de Moura A. F., Truzzi D. R., Nascimento O. R., Simonneau A., Marques Netto C. G. C.
Dalton Transactions 2023, 52(32), 11254-11264.
http://dx.doi.org/10.1039/D3DT01091K
https://hal.science/hal-04197074
Oxidative damages on the Alzheimer’s related-Aβ peptide alters its ability to assemble
Cheignon C., Collin F., Sabater L., Hureau C.
Antioxidants 2023, 12(2), 472/1-20.
https://doi.org/10.3390/antiox12020472
https://hal.science/hal-03988839
Ability of azathiacyclen ligands to stop Cu(Aβ)-induced production of reactive oxygen species: [3N1S] is the right donor set
Malikidogo K. P., Drommi M., Atrián-Blasco E., Hormann J., Kulak N., Esmieu C., Hureau C.
Chemistry – A European Journal 2023, 29(14), e202203667/1-11.
https://doi.org/10.1002/chem.202203667
https://hal.archives-ouvertes.fr/hal-03931017
New antiplasmodial 4-amino-thieno[3,2-d]pyrimidines with improved intestinal permeability and microsomal stability
Lagardère P., Mustière R., Amanzougaghene N., Hutter S., Casanova M., Franetich J.-F., Tajeri S., Malzert-Fréon A., Corvaisier S., Azas N., Vanelle P., Verhaeghe P., Primas N., Mazier D., Masurier N., Lisowski V.
European Journal of Medicinal Chemistry 2023, 249, 115115/1-12.
https://doi.org/10.1016/j.ejmech.2023.115115
https://hal.science/hal-04503268
2022
Development of Cu(ii)-specific peptide shuttles capable of preventing Cu–amyloid beta toxicity and importing bioavailable Cu into cells
Okafor M., Gonzalez P., Ronot P., El Masoudi I., Boos A., Ory S., Chasserot-Golaz S., Gasman S., Raibaut L., Hureau C., Vitale N., Faller P.
Chemical Science 2022, 13(40), 11829-11840.
http://dx.doi.org/10.1039/D2SC02593K
https://hal.science/hal-03876781
Synthesis, characterization and superoxide dismutase activity of a biomimetic Mn(III) complex covalently anchored to mesoporous silica
Richezzi M., Palopoli C., Pellegri N., Hureau C., Signorella S. R.
Journal of Inorganic Biochemistry 2022, 237, 112026/1-10.
https://doi.org/10.1016/j.jinorgbio.2022.112026
https://hal.science/hal-04669029
Improved emission of Yb(iii) ions in triazacyclononane-based macrocyclic ligands compared to cyclen-based ones
Kiraev S. R., Mathieu E., Kovacs D., Wells J. A. L., Tomar M., Andres J., Borbas K. E.
Dalton Transactions 2022, 51(43), 16596-16604.
http://dx.doi.org/10.1039/D2DT02266D
https://hal.science/hal-04297267
Sensitization pathways in NIR-emitting Yb(III) complexes bearing 0, +1, +2, or +3 charges
Mathieu E., Kiraev S. R., Kovacs D., Wells J. A. L., Tomar M., Andres J., Borbas K. E.
Journal of the American Chemical Society 2022, 144(46), 21056-21067.
https://doi.org/10.1021/jacs.2c05813
https://hal.science/hal-04297298
Deciphering RNA G-quadruplex function during the early steps of HIV-1 infection
Amrane S., Jaubert C., Bedrat A., Rundstadler T., Recordon-Pinson P., Aknin C., Guédin A., De Rache A., Bartolucci L., Diene I., Lemoine F., Gascuel O., Pratviel G., Mergny J.-L., Andreola M.-L.
Nucleic Acids Research 2022, 50(21), 12328-12343.
https://doi.org/10.1093/nar/gkac1030
https://hal.science/hal-03874577
Sequence–activity relationship of ATCUN peptides in the context of Alzheimer’s disease
Lefèvre M., Malikidogo K. P., Esmieu C., Hureau C.
Molecules 2022, 27(22), 7903/1-17.
https://doi.org/10.3390/molecules27227903
https://hal.archives-ouvertes.fr/hal-03875481
Effect of N-alkylation in N-carboxyanhydride (NCA) ring-opening polymerization kinetics
Salas-Ambrosio P., Tronnet A., Badreldin M., Ji S., Lecommandoux S., Harrisson S., Verhaeghe P., Bonduelle C.
Polymer Chemistry 2022, 13, 6149-6161.
http://dx.doi.org/10.1039/D2PY00985D
https://hal.archives-ouvertes.fr/hal-03813069
Synthesis, structure, and biologic activity of some copper, nickel, cobalt, and zinc complexes with 2-formylpyridine N4-allylthiosemicarbazone
Graur V., Chumakov Y., Garbuz O., Hureau C., Tsapkov V., Gulea A.
Bioinorganic Chemistry and Applications 2022, 2022, 2705332/1-18.
https://doi.org/10.1155/2022/2705332
https://hal.archives-ouvertes.fr/hal-03807954
Why the Ala-His-His peptide is an appropriate scaffold to remove and redox silence copper ions from the Alzheimer’s-related Aβ peptide
Gonzalez P., Sabater L., Mathieu E., Faller P., Hureau C.
Biomolecules 2022, 12(10), 1327/1-14.
https://doi.org/10.3390/biom12101327
https://hal.archives-ouvertes.fr/hal-03799992
Improving aqueous solubility and in vitro pharmacokinetic properties of the 3-nitroimidazo[1,2-a]pyridine antileishmanial pharmacophore
Paoli-Lombardo R., Primas N., Bourgeade-Delmas S., Hutter S., Sournia-Saquet A., Boudot C., Brenot E., Castera-Ducros C., Corvaisier S., Since M., Malzert-Fréon A., Courtioux B., Valentin A., Verhaeghe P., Azas N., Rathelot P., Vanelle P.
Pharmaceuticals 2022, 15(8), 998/1-26.
https://doi.org/10.3390/ph15080998
https://hal.archives-ouvertes.fr/hal-03755519
Drug screening approach against mycobacterial fatty acyl-AMP ligase FAAL32 renews the interest of the salicylanilide pharmacophore in the fight against tuberculosis
Le N.-H., Constant P., Tranier S., Nahoum V., Guillet V., Maveyraud L., Daffé M., Mourey L., Verhaeghe P., Marrakchi H.
Bioorganic & Medicinal Chemistry 2022, 71116938/1-7.
https://doi.org/10.1016/j.bmc.2022.116938
https://hal.archives-ouvertes.fr/hal-03758907
Synthesis of antiplasmodial 2-aminothieno[3,2-d]pyrimidin-4(3H)-one analogues using the scaffold hopping strategy
Mustière R., Lagardère P., Hutter S., Dell’Orco V., Amanzougaghene N., Tajeri S., Franetich J.-F., Corvaisier S., Since M., Malzert-Fréon A., Masurier N., Lisowski V., Verhaeghe P., Mazier D., Azas N., Vanelle P., Primas N.
European Journal of Medicinal Chemistry 2022, 241, 114619/1-13.
https://doi.org/10.1016/j.ejmech.2022.114619
https://hal.archives-ouvertes.fr/hal-03737417
4-Substituted Thieno[3,2-d]pyrimidines as Dual-Stage Antiplasmodial Derivatives
Lagardère P., Mustière R., Amanzougaghene N., Hutter S., Franetich J.-F., Azas N., Vanelle P., Verhaeghe P., Primas N., Mazier D., Masurier N., Lisowski V.
Pharmaceuticals 2022, 15(7), 820/1-18.
https://doi.org/10.3390/ph15070820
https://hal.archives-ouvertes.fr/hal-03725749
Pd-catalyzed C–C and C–N cross-coupling reactions in 2-aminothieno[3,2-d]pyrimidin-4(3H)-one series for antiplasmodial pharmacomodulation
Mustière R., Lagardère P., Hutter S., Deraeve C., Schwalen F., Amrane D., Masurier N., Azas N., Lisowski V., Verhaeghe P., Mazier D., Vanelle P., Primas N.
RSC Advances 2022, 12(31), 20004-20021.
http://dx.doi.org/10.1039/D2RA01687G
https://hal.archives-ouvertes.fr/hal-03726141
Star-like poly(peptoid)s with selective antibacterial activity
Salas-Ambrosio P., Tronnet A., Badreldin M., Reyes L., Since M., Bourgeade-Delmas S., Dupuy B., Verhaeghe P., Bonduelle C.
Polymer Chemistry 2022, 13(5), 600-612.
http://dx.doi.org/10.1039/D1PY01529J
https://hal.archives-ouvertes.fr/hal-03559890
Versatile activity of a copper(II) complex bearing a N4-tetradentate schiff base ligand with reduced oxygen species
Richezzi M., Ferreyra J., Puzzolo J., Milesi L., Palopoli C. M., Moreno D. M., Hureau C., Signorella S. R.
European Journal of Inorganic Chemistry 2022, (8), e202101042/1-11.
https://doi.org/10.1002/ejic.202101042
https://hal.archives-ouvertes.fr/hal-03549477
Role of Metal Ions in Alzheimer’s Disease: Mechanistic Aspects Contributing to Neurotoxicity
Hureau C.
in Alzheimer’s Disease: Recent Findings in Pathophysiology, Diagnostic and Therapeutic Modalities Govindaraju T. (Ed.). The Royal Society of Chemistry: Cambridge, 2022, pp. 170-192. (978-1-83916-230-5).
https://doi.org/10.1039/9781839162732-00170
https://hal.archives-ouvertes.fr/hal-03528657
Keggin-type polyoxometalates as Cu(II) chelators in the context of Alzheimer’s disease
Atrián-Blasco E., de Cremoux L., Lin X., Mitchell-Heggs R., Sabater L., Blanchard S., Hureau C.
Chemical Communications 2022, 58(14), 2367-2370.
http://dx.doi.org/10.1039/D1CC05792H
https://hal.archives-ouvertes.fr/hal-03547481
2021
Stefaniak E., Atrian-Blasco E., Goch W., Sabater L., Hureau C., Bal W.
Chemistry – A European Journal 2021, 27(8), 2798-2809.
https://doi.org/10.1002/chem.202004484
https://hal.archives-ouvertes.fr/hal-03203165v1
Copper imbalance in Alzheimer’s disease and its link with the amyloid hypothesis: Towards a combined clinical, chemical, and genetic etiology
Squitti R., Faller P., Hureau C., Granzotto A., White A. R., Kepp K. P.
Journal of Alzheimer’s Disease 2021, 83(1), 23-41.
https://doi.org/10.3233/jad-201556
https://hal.archives-ouvertes.fr/hal-03331612
Synthetic polypeptide polymers as simplified analogues of antimicrobial peptides
Salas-Ambrosio P., Tronnet A., Verhaeghe P., Bonduelle C.
Biomacromolecules 2021, 22(1), 57-75.
https://doi.org/10.1021/acs.biomac.0c00797
https://hal.archives-ouvertes.fr/hal-02931945
Cyclic poly(α-peptoid)s by lithium bis(trimethylsilyl)amide (LiHMDS)-mediated ring-expansion polymerization: Simple access to bioactive backbones
Salas-Ambrosio P., Tronnet A., Since M., Bourgeade-Delmas S., Stigliani J.-L., Vax A., Lecommandoux S., Dupuy B., Verhaeghe P., Bonduelle C.
Journal of the American Chemical Society 2021, 143(10), 3697-3702.
https://doi.org/10.1021/jacs.0c13231
https://hal.archives-ouvertes.fr/hal-03173586
Gold(III) porphyrins: Synthesis and interaction with G-quadruplex DNA
Rundstadler T., Mothes E., Amrane S., Stigliani J.-L., Verhaeghe P., Pratviel G.
Journal of Inorganic Biochemistry 2021, 223, 111551/1-10.
https://doi.org/10.1016/j.jinorgbio.2021.111551
https://hal.archives-ouvertes.fr/hal-03312437
Concentration-dependent interactions of amphiphilic PiB- derivative metal complexes with amyloid peptides Aβ and amylin
Majdoub S., Garda Z., Oliveira A. C., Relich I., Pallier A., Lacerda S., Hureau C., Geraldes C. F. G. C., Morfin J.-F., Tóth É.
Chemistry – A European Journal 2021, 27, 2009-2020.
https://doi.org/10.1002/chem.202004000
https://hal.archives-ouvertes.fr/hal-03011656
SARS-CoV-2 Nsp3 unique domain SUD interacts with guanine quadruplexes and G4-ligands inhibit this interaction
Lavigne M., Helynck O., Rigolet P., Boudria-Souilah R., Nowakowski M., Baron B., Brülé S., Hoos S., Raynal B., Guittat L., Beauvineau C., Petres S., Granzhan A., Guillon J., Pratviel G., Teulade-Fichou M.-P., England P., Mergny J.-L., Munier-Lehmann H.
Nucleic Acids Research 2021, 49(13), 7695-7712.
https://doi.org/10.1093/nar/gkab571
https://hal.archives-ouvertes.fr/hal-03281130
Synthesis, characterization, and biological activity of novel 3d metal coordination compounds with 2-acetylpyridine N4-allyl-S-methylisothiosemicarbazone
Graur V., Usataia I., Bourosh P., Kravtsov V., Garbuz O., Hureau C., Gulea A.
Applied Organometallic Chemistry 2021, 35(4), e6172/1-17.
https://doi.org/10.1002/aoc.6172
https://hal.archives-ouvertes.fr/hal-03412897
Reproducibility problems of amyloid-β self-assembly and how to deal with them
Faller P., Hureau C.
Frontiers in chemistry 2021, 8611227/1-7.
https://doi.org/10.3389/fchem.2020.611227
https://hal.archives-ouvertes.fr/hal-03203136
Impact of N-truncated Aβ peptides on Cu- and Cu(Aβ)-generated ROS: CuI matters!
Esmieu C., Ferrand G., Borghesani V., Hureau C.
Chemistry – A European Journal 2021, 27(5), 1777-1786.
https://doi.org/10.1002/chem.202003949
https://hal.archives-ouvertes.fr/hal-03383515
Unexpected trends in copper removal from Aβ peptide: when less ligand is better and Zn helps
Esmieu C., Balderrama-Martínez-Sotomayor R., Conte-Daban A., Iranzo O., Hureau C.
Inorganic Chemistry 2021, 60(2), 1248-1256.
https://doi.org/10.1021/acs.inorgchem.0c03407
https://hal.archives-ouvertes.fr/hal-03203129
Hybrid bis-histidine phenanthroline-based ligands to lessen Aβ-bound Cu ROS production: An illustration of Cu(I) significance
Drommi M., Rulmont C., Esmieu C., Hureau C.
Molecules 2021, 26(24), 7630/1-15.
https://doi.org/10.3390/molecules26247630
https://hal.archives-ouvertes.fr/hal-03547406
Voltammetric studies of selected porphyrin G-quadruplex ligands and their interaction with DNA in solution and at the mercury electrode surface
Dobrovodsky D., Danhel A., Mothes-Martin E., Pratviel G., Mergny J.-L., Fojta M.
Electrochimica Acta 2021, 394, 139151/1-11.
https://doi.org/10.1016/j.electacta.2021.139151
https://hal.archives-ouvertes.fr/hal-03357381
A new thienopyrimidinone chemotype shows multistage activity against Plasmodium falciparum, including artemisinin-resistant parasites
Bosson-Vanga H., Primas N., Franetich J.-F., Lavazec C., Gomez L., Ashraf K., Tefit M., Soulard V., Dereuddre-Bosquet N., Le Grand R., Donnette M., Mustière R., Amanzougaghene N., Tajeri S., Suzanne P., Malzert-Fréon A., Rault S., Vanelle P., Hutter S., Cohen A., Snounou G., Roques P., Azas N., Lagardère P., Lisowski V., Masurier N., Nguyen M., Paloque L., Benoit-Vical F., Verhaeghe P., Mazier D., Muralidharan V.
Microbiology Spectrum 2021, 9(2), e00274-21/1-19.
https://doi.org/10.1128/Spectrum.00274-21
https://hal.archives-ouvertes.fr/hal-03363796
Measurement of interpeptidic CuII exchange rate constants of CuII-Amyloid-β complexes to small peptide motifs by tryptophan fluorescence quenching
Beuning C. N., Zocchi L. J., Malikidogo K. P., Esmieu C., Dorlet P., Crans D. C., Hureau C.
Inorganic Chemistry 2021, 60(11), 7650-7659.
https://doi.org/10.1021/acs.inorgchem.0c03555
https://hal.archives-ouvertes.fr/hal-03380483
Solid-state and solution characterizations of [(TMPA)Cu(II)(SO3)] and [(TMPA)Cu(II)(S2O3)] complexes: Application to sulfite and thiosulfate fast detection
Berthonnaud L., Esmieu C., Mallet-Ladeira S., Hureau C.
Journal of Inorganic Biochemistry 2021, 225, 111601/1-9.
https://doi.org/10.1016/j.jinorgbio.2021.111601
https://hal.archives-ouvertes.fr/hal-03394471
A water-soluble peptoid chelator that can remove Cu2+ from amyloid-β peptides and stop the formation of reactive oxygen species associated with Alzheimer’s disease
Behar A. E., Sabater L., Baskin M., Hureau C., Maayan G.
Angewandte Chemie, International Edition 2021, 60(46), 24588-24597.
https://doi.org/10.1002/anie.202109758
https://hal.archives-ouvertes.fr/hal-03395343
Antiplasmodial 2-thiophenoxy-3-trichloromethyl quinoxalines target the apicoplast of Plasmodium falciparum
Amrane D., Primas N., Arnold C.-S., Hutter S., Louis B., Sanz-Serrano J., Azqueta A., Amanzougaghene N., Tajeri S., Mazier D., Verhaeghe P., Azas N., Botté C., Vanelle P.
European Journal of Medicinal Chemistry 2021, 224, 113722/1-14.
https://doi.org/10.1016/j.ejmech.2021.113722
https://hal.archives-ouvertes.fr/hal-03323972
2-phenoxy-3-trichloromethylquinoxalines are antiplasmodial aerivatives with activity against the apicoplast of Plasmodium falciparum
Amrane D., Arnold C.-S., Hutter S., Sanz-Serrano J., Collia M., Azqueta A., Paloque L., Cohen A., Amanzougaghene N., Tajeri S., Franetich J.-F., Mazier D., Benoit-Vical F., Verhaeghe P., Azas N., Vanelle P., Botté C., Primas N.
Pharmaceuticals 2021, 14(8), 724/1-24.
https://doi.org/10.3390/ph14080724
https://hal.archives-ouvertes.fr/hal-03323862
Partnerships
Click here to consult the partners
International collaborators
- S. Signorella, University of Rosario, Argentina
Studies of Mn complexes, enzyme mimes (catalase and superoxide dismutase and plant oxygen release center). Collaboration supported by the PICS program of CNRS for the 2016-2018 period.
- W. Bal, University of Wroclaw, Poland
Analytical studies of Cu(II) coordination to peptides and aggregation of truncated peptides.
- D. Valensin, University of Siena, Italy
Study of the coordination of Cu(II) ions to the α-synuclein protein involved in Parkinson’s disease.
- D. Crans, University of Colorado,
Kinetic studies of metal ion exchange between peptides. Collaboration supported by a Chateaubriand grant for C. Beuning.
- G. Maayan, Technion University, Israel.
Peptoid ligands as Cu(II) chelators in Alzheimer’s disease.
French Collaborators
- K. Reybier, IRD, University of Toulouse
Study of oxidative stress generated by Cu(Aβ) on cells.
- P. Genevaux, Laboratory of Microbiology and Molecular Genetics, Toulouse.
Study of chaperone proteins of amyloid-β peptide aggregation.
- E. Anxolabéhère-Mallart, University D. Diderot, Paris
Studies of Mn complexes, enzyme mimes (catalase and superoxide dismutase)
- P. Delangle, Ion Recognition and Coordination Chemistry Laboratory, CEA Grenoble
Study of Cu(I/II) chelators in the context of Alzheimer’s disease
- C. Policar, ENS Ulm, Paris
Study of SOD mimics against oxidative stress in Alzheimer’s disease.
- N. Delsuc, ENS Ulm, Paris
Study of Cu(II) peptide complexes with SOD activity
- R. Tripier and Dr. Maryline Beyler, UMR 6521, Université de Bretagne Occidentale.
Study of macrocyclic Cu(II) chelators in the context of Alzheimer’s disease.
- O. Iranzo, Institute of Molecular Sciences of Marseille, UMR 7313, University of Aix – Marseille.
Study of pseudo-peptide chelators of Cu(II) in the context of Alzheimer’s disease.
- P. Faller, Institut de Chimie de Strasbourg, UMR7177
Traceable Cu(II) metallophores as a therapeutic approach in Alzheimer’s disease.
- S. Blanchard, Institut Parisien de Chimie Moléculaire, UMR 8232
Study of polyoxometallates in Alzheimer’s disease.
- S. Hamdi, Human Fertility Research Group, EA 3694 of Paul-Sabatier University
Study of the role of metal ions in the aggregation of SEMS peptides involved in fertility
- E. Toth, Molecular Biophysics Center, UPR 4301, Orléans.
Markers of amylin aggregation involved in type II diabetes.
- Marc Blanchard, Geosciences Environnement Toulouse, Observatoire Midi-Pyrénées.
Use of stable isotopes of Cu and Zn ions to study Aβ assembly.
- O. Maury, F. Riobé, Chemistry Laboratory, ENS Lyon
Complexes for modulation and monitoring of amyloid aggregation.
- G. Lippens, LISBP, INSA-Toulouse
Study of phosphorus chaperones of Tau proteins
- O. Cuvillier, IPBS, UMR 5089
Study of cytotoxicity of Cu(Aβ) and restoration of cell survival.
- E. Benoist, SPCMIB, UMR 5068
Re(CO)3 complexes for amyloid imaging.
- N. Giraud, Université de Paris, UMR CNRS 8601
NMR study of Aβ interaction with external partners.
- F. Bedos and C. Bacqué, SPCMIB, UMR 5068
(Clickable) ligands targeting Cu(II) in the Alzheimer’s context.
Funding
Click here to consult the funders
LnZYME, ANR,
2023-2027
Lanthano-peptides as mimics of lanthanide methanol dehydrogenase enzyme
PI : Emilie Mathieu
Amyl-in-AD, MITI CNRS,
2023-2026
Is amylin the real villain in Alzheimer’s disease ?
PI : Christelle Hureau & Nicolas Vitale
MASAI, ANR,
2022-2026
Metal-based agents for selective amyloid imaging.
PI : Eva Tòth, Partner : Christelle Hureau
Read more: https://anr.fr/Project-ANR-22-CE44-0002
DREAMY, ANR 2023-2027
Deciphering early steps of self-assembly of amyloid forming peptides
PI : Nicolas Giraud, Partner : Christelle Hureau
Read more: https://anr.fr/Project-ANR-22-CE29-0026
SUPRAMY, ANR PRC 2021-2025
Polyanions to tune the supramolecular assembly of amyloid-forming peptides (SUPRAMY)
PI : Christelle Hureau
Read more: https://anr.fr/Project-ANR-21-CE06-0030
COPPERATION, ANR JCJC 2020-2024
Fluorescent ligands: for the rational design of ligands targeting copper ions in Alzheimer’s disease
PI : Charlène Esmieu
Read more: https://anr.fr/Projet-ANR-20-CE07-0009
ANRS, Emerging infectious diseases
2020 – 2023
Function and targeting of G-quadruplexes RNAs from HIV-1
PI : Dr Samir AMRANE (Institut Européen de Chimie Biologie, IECB, Bordeaux),
Partner: Geneviève Pratviel
DIVA, ANR 2016
2016 – 2021
Diabetes Imaging by Visualizing Amylin with Metal-based Probes
PI: Dr. E. Toth (CBM, Orléans), Partner: Christelle Hureau
Read more: https://anr.fr/Project-ANR-16-CE18-0022
CASPER, Fondation France-Alzheimer,
2016-2018
Dynamic Combinatorial Synthesis of Optical Molecular Markers of the Aggregated Amyloid-ß Peptide
PI: B. Mestre-Voegtlé
ALzINK, ERC (European Research Council) StG
2015-2020
Alzheimer’s disease & Zinc : the missing link ?
PI: Christelle HUREAU
Read more: https://cordis.europa.eu/project/id/638712
AlzPEPS, Fellowship USIAS
2015-2017
Peptides as Cu shuttles
PI: Christelle Hureau & Peter Faller (UMR 7177, Strasbourg)
Recoligo, France-Alzheimer,
2014-2017
Targeting amyloides-ß oligomers
PI: Christelle Hureau
AlzABox, ANR Blanc
2013-2017
Oxidation of the Aβ peptide and consequences in the aetiology of Alzheimer’s Disease
PI: Fabrice Collin ; Partner: Christelle Hureau
Read more: https://anr.fr/Projet-ANR-13-BSV5-0016
Neurometals, ANR Blanc
2009-2013
Impact of metallic ion in Alzheimer’s disease
PI: Peter Faller
Read more: https://anr.fr/Projet-ANR-09-BLAN-0090
PICS, CNRS-CONICET
2016-2018
Mn-based biosinpired Water-Oxidizing Complex
PI: Christelle Hureau
Alumnae
Click here to view
- Antoine TRONNET
2018-2022 – Polymeric analog of antimicrobial peptides with anti-Clostridium difficile therapeutic potential - Tiffany RUNDSTADLER
2018-2022 – G-quadruplex ligands of nucleic acids: modeling, synthesis and antiviral activity - Xudong LIN
2018-2022 – Synthesis and studies of compounds targeting the aggregation of amyloid-beta peptide - Raul BALDERRAMA
2019 – Peptidomimetic ligands for the chelation of Cu(II) - Ewelina STEFANIAK
2016-2017 – Coordination of Cu(II) ions to peptides for SOD activity - Amandine VINCENT
2018-2019 – Co-aggregation of amyloid peptides - Sara AYALA
2014-2018 – Role of Zn ions and chaperone proteins in the aggregation of different forms of amyloid-β peptide - Alexandre POCINHO
2014-2018 – Optimization of molecules for recognition of oligomeric forms of amyloid-β peptides - Valentina BORGHESANI
2015-2018 – Impact of Zn ions in the aggregation of different forms of amyloid-β peptide - Amandine CONTE-DABAN
2014-2017 – Role of Cu and Zn ions in the aggregation and toxicity of metal complexes of amyloid-β peptide: mechanistic and therapeutic aspects
LCC
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France