LCC
Overview
The team consists of chemists, biologists, pharmacists and clinicians. We have brought these skills together to develop drug candidates for cancer treatment based on organometallic complexes.
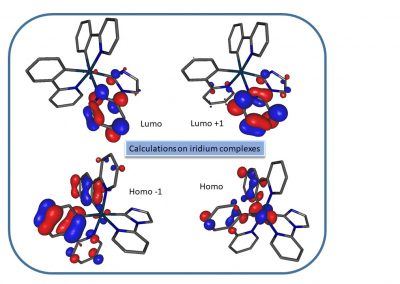
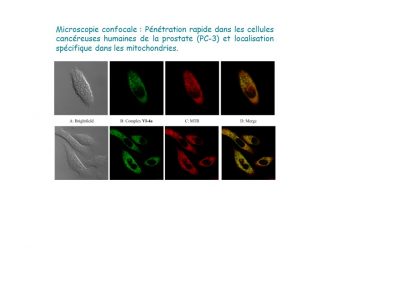
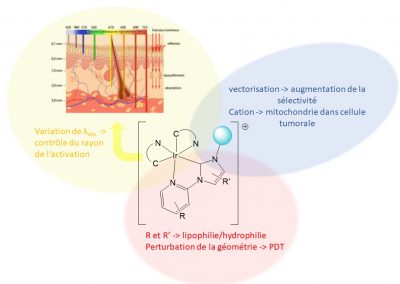
We are mainly developing gold and iridium complexes and are investigating their cytotoxic properties, modes of action and, in the case of iridium, their potential as a photosensitiser for application in photodynamic therapy.
Members of the team

CUVILLIER Olivier

GORNITZKA Heinz

DERAEVE Céline

GARNIER Maritie

HEMMERT Catherine

LAL Kanhaya

LIANG Zhengcheng

MADDELEIN Marie-Lise

MALAVAUD Bernard

PELTRET Thomas

SALIS Chloé

STIGLIANI Jean-Luc
No Results Found
Research topics
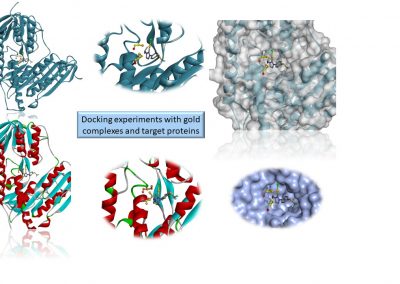
Gold complexes for biomedical applications
Iridium complexes for photodynamic therapy
N-heterocyclic derivatives for biomedical applications
Team news
Highlight 2021 Team 0
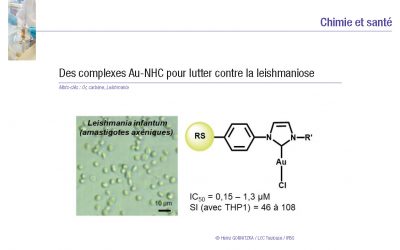
Leishmaniasis is one of the five major parasitic diseases with vectorial transmission, with an estimated annual incidence of one million cases.
Publications
2025
New cyclopeptide and γ-butyrolactone derivatives from an endophytic strain of Cophinforma mamane
Litot C., Vansteelandt M., Ortiz S., Barakat F., Crossay E., Titton D., Bourgeade-Delmas S., Amasifuen C., Graton J., Jacquemin D., Stigliani J.-L., Haddad M., Fabre N.
ACS Omega 2025, 10(21), 21738-21746.
10.1021/acsomega.5c01292 – hal-05121071
Mechanochemistry to form substituted imidazoles, imidazolium salts and NHC–Gold complexes with fluorine-containing groups
Salis C., Mohammedi S., Turazza L., Blandin Y., Garnier M., Hemmert C., Baltas M., Gornitzka H.
Molecules 2025, 30(3), 522/1-16.
10.3390/molecules30030522 – hal-04931989
2024
Electrophilic behavior of the “nucleophilic” pyramidane: reactivity of Ge-pyramidane towards organolithium reagents
Lee V. Y., Wang J., Sasamori T., Gapurenko O. A., Minyaev R. M., Minkin V. I., Takeuchi K., Fukaya N., Gornitzka H.
Chemistry – A European Journal 2024, 30(45), e202401806/1-5.
https://doi.org/10.1002/chem.202401806
https://hal.science/hal-04804011
Mono- and Dinuclear 1-(2-Pyridyl)-4-phenyl-1,2,3-triazole-Based Ir(III) and Rh(III) Complexes
Moreno-Latorre M., de la Torre M. C., Gornitzka H., Hemmert C., Sierra M. A.
Organometallics 2024, 43(10), 1128-1136.
https://doi.org/10.1021/acs.organomet.4c00079
https://hal.science/hal-04669053
Rationally designed Cu(I) ligand to prevent CuAβ-generated ROS production in the Alzheimer’s disease context
Rulmont C., Stigliani J.-L., Hureau C., Esmieu C.
Inorganic Chemistry 2024, 63(5), 2340-2351.
https://doi.org/10.1021/acs.inorgchem.3c02693
https://hal.science/hal-04669053
2023
X-ray Crystallography of Organogermanium Compounds
Hemmert C., Gornitzka H.
in Organogermanium Compounds, V. Y. Lee (Ed.), John Wiley & Sons: Hoboken, 2023, vol. 2, pp. 667-743. (978-1-1196-1352-7).
https://doi.org/10.1002/9781119613466.ch16
https://hal.science/hal-04244894
Pharmacological characterization of [18F]-FNM and evaluation of NMDA receptors activation in a rat brain injury model
Beaurain M., Talmont F., Pierre D., Péran P., Boucher S., Hitzel A., Rols M.-P., Cuvillier O., Payoux P., Salabert A.-S.
Molecular Imaging and Biology 2023, 25(4), 692-703.
https://doi.org/10.1007/s11307-023-01811-y
https://hal.science/hal-04244094
Hybrid bicyclo[1.1.0]butanes [(R4Si3)CH2] and [(R4Si2Ge)CH2] (R = SiMetBu2): Synthesis and thermal isomerization to alkyl-substituted cyclopropenes [R3Si3]CH2R and [R3Si2Ge]CH2R
Lee V. Y., Yasuda H., Gapurenko O. A., Minyaev R. M., Gornitzka H., Sekiguchi A.
Organometallics 2023, 42(18), 2514-2521.
https://doi.org/10.1021/acs.organomet.2c00599
https://hal.science/hal-04175751
Daphnanes diterpenes from the latex of Hura crepitans L. and their PKCζ-dependent anti-proliferative activity on colorectal cancer cells
Crossay E., Jullian V., Trinel M., Sagnat D., Hamel D., Groppi E., Rolland C., Stigliani J.-L., Mejia K., Cabanillas B. J., Alric L., Buscail E., El Kalamouni C., Mavingui P., Deraison C., Racaud-Sultan C., Fabre N.
Bioorganic & Medicinal Chemistry 2023, 90, 117366/1-17.
https://doi.org/10.1016/j.bmc.2023.117366
https://hal.science/hal-04244338
N-heterocyclic carbene-iridium complexes as photosensitizers for in vitro photodynamic therapy to trigger non-apoptotic cell death in cancer cells
Wang X., Zhang C., Madji R., Voros C., Mazères S., Bijani C., Deraeve C., Cuvillier O., Gornitzka H., Maddelein M.-L., Hemmert C.
Molecules 2023, 28(2), 691/1-29.
https://doi.org/10.3390/molecules28020691
https://hal.science/hal-03870710
2022
Sphingosine kinase-1 is overexpressed and correlates with hypoxia in osteosarcoma: Relationship with clinicopathological parameters
Gomez-Brouchet A., Illac C., Ledoux A., Fortin P.-Y., de Barros S., Vabre C., Despas F., Peries S., Casaroli C., Bouvier C., Aubert S., de Pinieux G., Larousserie F., Galmiche L., Talmont F., Pitson S., Maddelein M.-L., Cuvillier O.
Cancers 2022, 14 (3), 499/1-17.
https://doi.org/10.3390/cancers14030499
https://hal.science/hal-03312272v2
Sphingosine 1-phosphate receptor 5 (S1P5) deficiency promotes proliferation and immortalization of mouse embryonic fibroblasts
Talmont F., Mitri E., Dozier C., Besson A., Cuvillier O., Hatzoglou A.
Cancers 2022, 14 (7), 1661/1-20.
https://doi.org/10.3390/cancers14071661
https://hal.science/hal-03808905v1
Shvo-type metal–ligand cooperative catalysts: Tethered η5-oxocyclohexadienyl ruthenium complexes
Puig E., Verron R., Kechaou-Perrot M., Vendier L., Gornitzka H., Miqueu K., Sotiropoulos J.-M., Fischmeister C., Sutra P., Igau A.
Organometallics 2022, 41(11), 1391-1402.
https://doi.org/10.1021/acs.organomet.2c00123
https://hal.archives-ouvertes.fr/hal-03715383
Pd-catalyzed C–C and C–N cross-coupling reactions in 2-aminothieno[3,2-d]pyrimidin-4(3H)-one series for antiplasmodial pharmacomodulation
Mustière R., Lagardère P., Hutter S., Deraeve C., Schwalen F., Amrane D., Masurier N., Azas N., Lisowski V., Verhaeghe P., Mazier D., Vanelle P., Primas N.
RSC Advances 2022, 12(31), 20004-20021.
http://dx.doi.org/10.1039/D2RA01687G
https://hal.archives-ouvertes.fr/hal-03726141
Investigation of direct and retro chromone-2-carboxamides based analogs of Pseudomonas aeruginosa quorum sensing signal as new anti-biofilm agents
Trognon J., Vera G., Rima M., Stigliani J.-L., Amielet L., El Hage S., Lajoie B., Roques C., El Garah F.
Pharmaceuticals 2022, 15(4), 417/1-26.
https://doi.org/10.3390/ph15040417
https://hal.archives-ouvertes.fr/hal-03669961
1-Chloroalumole
Lee V. Y., Sugasawa H., Gapurenko O. A., Minyaev R. M., Minkin V. I., Gornitzka H., Sekiguchi A.
Organometallics 2022, 41(4), 467-471.
https://doi.org/10.1021/acs.organomet.1c00702
https://hal.archives-ouvertes.fr/hal-03660785
Phosphatetrasilatricyclo[2.1.0.02,5]pentane
Lee V. Y., Meguro T., Gapurenko O. A., Minyaev R. M., Minkin V. I., Gornitzka H., Sekiguchi A.
Mendeleev Communications 2022, 32(1), 33-34.
https://doi.org/10.1016/j.mencom.2022.01.009
https://hal.archives-ouvertes.fr/hal-03660695
β-Lactam and penicillin substituted mesoionic metal carbene complexes
Avello M. G., Moreno-Latorre M., de la Torre M. C., Casarrubios L., Gornitzka H., Hemmert C., Sierra M. A.
Organic & Biomolecular Chemistry 2022, 20(13), 2651-2660.
http://dx.doi.org/10.1039/D2OB00216G
https://hal.archives-ouvertes.fr/hal-03660770
2021
Inhibition of sphingosine 1-phosphate protects mice against chondrocyte catabolism and osteoarthritis
Cherifi C., Latourte A., Vettorazzi S., Tuckermann J., Provot S., Ea H. K., Ledoux A., Casas J., Cuvillier O., Richette P., Ostertag A., Hay E., Cohen-Solal M.
Osteoarthritis and Cartilage 2021, 29 (9), 1335-1345.
https://doi.org/10.1016/j.joca.2021.06.001
https://hal.science/hal-04202734
Cyclic poly(α-peptoid)s by lithium bis(trimethylsilyl)amide (LiHMDS)-mediated ring-expansion polymerization: Simple access to bioactive backbones
Salas-Ambrosio P., Tronnet A., Since M., Bourgeade-Delmas S., Stigliani J.-L., Vax A., Lecommandoux S., Dupuy B., Verhaeghe P., Bonduelle C.
Journal of the American Chemical Society 2021, 143(10), 3697-3702.
https://doi.org/10.1021/jacs.0c13231
https://hal.archives-ouvertes.fr/hal-03173586
Gold(III) porphyrins: Synthesis and interaction with G-quadruplex DNA
Rundstadler T., Mothes E., Amrane S., Stigliani J.-L., Verhaeghe P., Pratviel G.
Journal of Inorganic Biochemistry 2021, 223, 111551/1-10.
https://doi.org/10.1016/j.jinorgbio.2021.111551
https://hal.archives-ouvertes.fr/hal-03312437
Novel molecule combinations and corresponding hybrids targeting artemisinin-resistant Plasmodium falciparum parasites
Ouji M., Nguyen M., Mustière R., Jimenez T., Augereau J.-M., Benoit-Vical F., Deraeve C.
Bioorganic & Medicinal Chemistry Letters 2021, 39, 127884/1-6.
https://doi.org/10.1016/j.bmcl.2021.127884
https://hal.archives-ouvertes.fr/hal-03170976
®-BINOL-6,6’-bistriflone: Shortened synthesis, characterization, and enantioselective catalytic applications
Mouhtady O., Castellan T., André-Barrès C., Gornitzka H., Fabing I., Saffon-Merceron N., Génisson Y., Gaspard H.
European Journal of Organic Chemistry 2021, 2021(48), 6674-6681.
https://doi.org/10.1002/ejoc.202101137
https://hal.archives-ouvertes.fr/hal-03511667
Titanium germylidenes
Lee V. Y., Sakai R., Takanashi K., Gapurenko O. A., Minyaev R. M., Gornitzka H., Sekiguchi A.
Angewandte Chemie, International Edition 2021, 60(8), 3951-3955.
https://doi.org/10.1002/anie.202015704
https://hal.archives-ouvertes.fr/hal-03203959
Chlorinated bianthrones from the cyanolichen Nephroma laevigatum
Lagarde A., Mambu L., Mai P.-Y., Champavier Y., Stigliani J.-L., Beniddir M. A., Millot M.
Fitoterapia 2021, 149, 104811/1-8.
https://doi.org/10.1016/j.fitote.2020.104811
https://hal.archives-ouvertes.fr/hal-03203919
Metallodrug profiling against SARS-CoV-2 target proteins identifies highly potent inhibitors of the S/ACE2 interaction and the Papain-like protease PLpro
Gil-Moles M., Türck S., Basu U., Pettenuzzo A., Bhattacharya S., Rajan A., Ma X., Büssing R., Wölker J., Burmeister H., Hoffmeister H., Schneeberg P., Prause A., Lippmann P., Kusi-Nimarko J., Hassell-Hart S., McGown A., Guest D., Lin Y., Notaro A., Vinck R., Karges J., Cariou K., Peng K., Qin X., Wang X., Skiba J., Szczupak Ł., Kowalski K., Schatzschneider U., Hemmert C., Gornitzka H., Milaeva E. R., Nazarov A. A., Gasser G., Spencer J., Ronconi L., Kortz U., Cinatl J., Bojkova D., Ott I.
Chemistry – A European Journal 2021, 27(71), 17928-17940.
https://doi.org/10.1002/chem.202103258
https://hal.archives-ouvertes.fr/hal-03413330
Synthesis, characterization, and antileishmanial activity of neutral gold(I) complexes with N-heterocyclic carbene ligands bearing sulfur-containing side arms
Ftouh S., Bourgeade-Delmas S., Belkadi M., Deraeve C., Hemmert C., Valentin A., Gornitzka H.
Organometallics 2021, 40(10), 1466-1473.
https://doi.org/10.1021/acs.organomet.1c00113
https://hal.archives-ouvertes.fr/hal-03233814
Partnerships
Click here to consult the partners
Alexis Valentin (Pharmadev-Toulouse)
Karine Reybier (Pharmadev-Toulouse)
Fatima El Garah (LGC-Toulouse)
Jean-Philippe Torré (LGC-Toulouse)
Nicolas Masurier (IBMM Montpellier)
Nicolas Primas (ICR Marseille)
Colin Bonduelle (LCPO Pessac)
Miguel A. Sierra (Madrid-Spain)
Maria Carmen de la Torre (Madrid-Spain)
Vladimir Ya Lee (Tsukuba-Japan)
Funding
Click here to consult the funders
ANR, Cancéropôle Grand Sud-Ouest, ARC, ARTP, La Ligue Contre Le Cancer.
Alumnae
Click here to view
Luca BOSELLI
Chen ZHANG
Alvaro FERNANDEZ ALVAREZ
Xue QIN
Xing WANG
LCC
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France