LCC
Electrochemistry department overview
Electrochemistry plays a role in many industrial or research fields. This discipline is interested in chemical reactions that involve an exchange of electrical charges between two substances. Electrochemistry thus opens up new possibilities for specific syntheses and the detection of compounds.
This electrochemistry department, unique in France, offers several benefits for your R&D projects in different scientific fields (materials, organic or mineral chemistry, etc).
Service members

SOURNIA-SAQUET Alix

MOREAU Alain
Equipments
Schematic representation for CV experiments
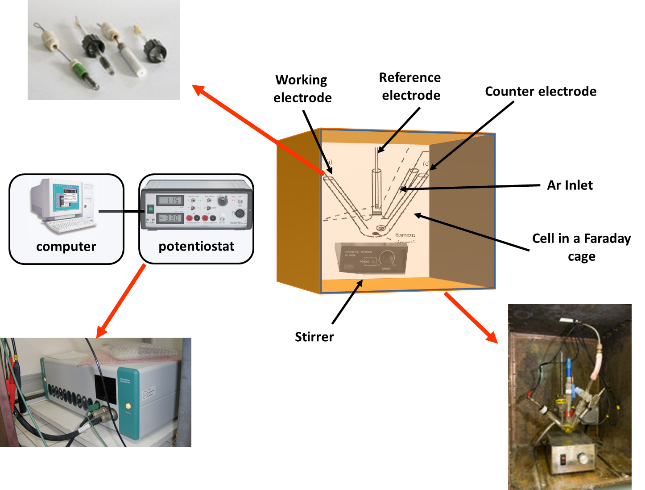
Potentiostats:
There are three additional potentiostats in the service

METROHM AUTOLAB PGSTAT100 (2002)
Caractéristiques :
Maximum current 250mA
Voltage compliance 100V

METROHM AUTOLAB PGSTATN302 (2012)
Caractéristiques :
Maximum current 2 A
Voltage compliance 30 V

METROHM AUTOLAB VIONIC (2021)
Caractéristiques :
Maximum current 6 A
Voltage compliance 50 V
EIS Frequency jusqu’à 10 MHz
Câblage pour boite à gants
Other materials:
- pH-meter – conductivity meter
- Cells:
- specific cell for working in an inert environment
- cell of different volumes (8ml to 100mL)

Significant choice of electrodes
- Choice of metals (Pt, Au, glassy carbon…)
- Different shapes and surfaces: microdisks of 10 µm with 3mm of diameter, wire or grid of some cm² of area.
- Rotating disk electrodes
- Specific electrodes for the powders: cavity microelectrodes, carbon powder microelectrode
- etc.


Experiments and specificities
- Potentiometric titration, pHmetry, conductivity
- the majority of the electrochemical experiments are made, in aqueous or non aqueous solvents, with ohmic drop compensation
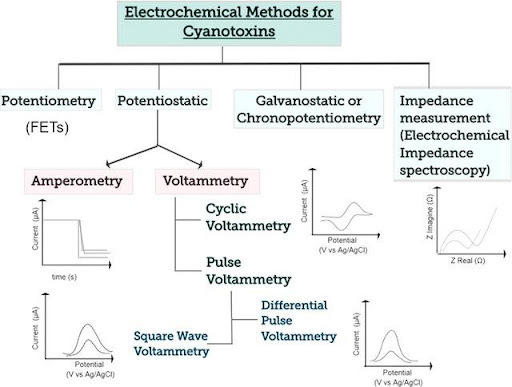
- Voltammetry cyclic or linear sweep voltammetry, normal or differential pulse voltammetry, square wave voltammetry, etc..
- Chrono methods: chronomaperometry , chronopotentiometry
Electrochemical studies on insoluble products with cavity microelectrode or carbon powder microelectrode
Services
The service provides equipment, staff, and know-how necessary for realizing different electrochemical techniques: characterization, kinetic studies, syntheses, and titrations (pHmetry, potentiometry, and conductimetry).
Eventually, the head of the department uses the data obtained and writes the results of scientific articles. She advises you to make an appointment to check the feasibility of your study. Following this interview, she will establish a detailed estimate according to your needs, and then she will propose a schedule of experiments.
The price of the study will depend on used products, the chosen electrochemical technique and, the estimated time to make the measurement.
Examples of the shape of curves obtained according to the different techniques:
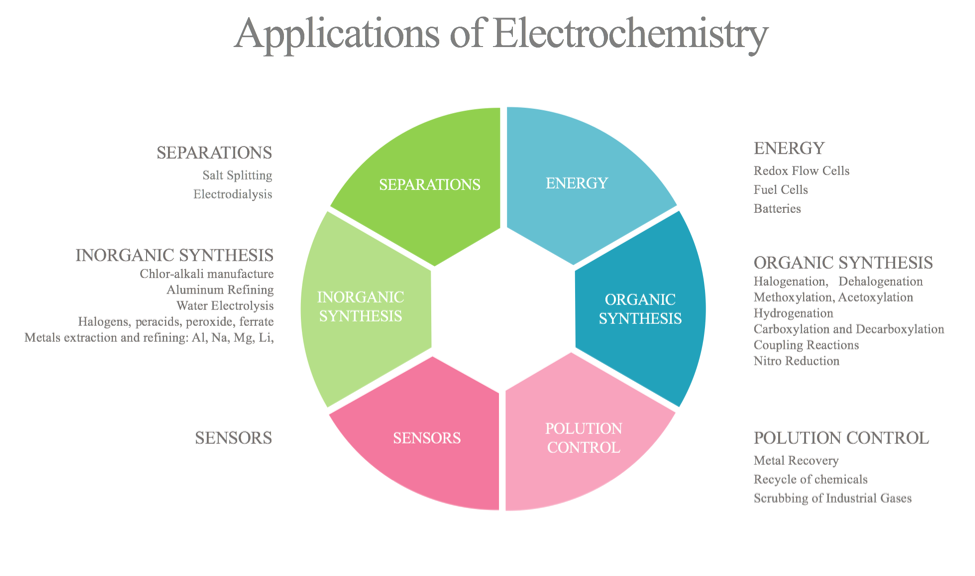
Applications of electrochemistry :
Publications
view the last publications
The effects of alkyl substitution on the aggregation of π conjugated dyes: spectroscopic study and modelling
Bardi B., Saquet A., Moreau A., Moineau-Chane Ching K., Terenziani F.
Physical Chemistry Chemical Physics 2024, 26(25), 17796-17808.
http://dx.doi.org/10.1039/D4CP01579G
https://hal.science/hal-04606301
Single electron reduction of NHC–CO2–borane compounds
Morales A., Gonçalves C., Sournia-Saquet A., Vendier L., Lledós A., Baslé O., Bontemps S.
Chemical Science 2024, 15(9), 3165-3173.
http://dx.doi.org/10.1039/D3SC06325A
https://hal.science/hal-04502219
Synthesis of new 5- or 7-substituted 3-nitroimidazo[1,2-a]pyridine derivatives using SNAr and palladium-catalyzed reactions to explore antiparasitic structure–activity relationships
Paoli-Lombardo R., Primas N., Bourgeade-Delmas S., Sournia-Saquet A., Castera-Ducros C., Jacquet I., Verhaeghe P., Rathelot P., Vanelle P.
Synthesis 2024, 56(8), 1297-1308.
http://doi.org/10.1055/a-2232-8113
https://hal.science/hal-04534726
From stilbenes to carbo-stilbenes: An encouraging prospect
Zhu C., Saquet A., Maraval V., Bijani C., Cui X., Poater A., Chauvin R.
Chemistry – A European Journal 2024, 30(26), e202400451/1-7.
https://doi.org/10.1002/chem.202400451
https://hal.science/hal-04532883
A mononuclear cobalt(III) carboxylate complex with a fully O-based coordination sphere: CoIII-O bond homolysis and controlled radical polymerisation from [Co(acac)2(O2CPh)]
Michelas M., Daran J.-C., Saquet A., Fliedel C., Poli R.
Dalton Transactions 2023, 52 (20), 6791-6798.
http://dx.doi.org/10.1039/D3DT00910F
https://hal.science/hal-04084576
Acetylacetonate ruthenium nitrosyls: A gateway to nitric oxide release in water under near-infrared excitation by two-photon absorption
Labra-Vázquez P., Mudrak V., Tassé M., Mallet-Ladeira S., Sournia-Saquet A., Malval J.-P., Lacroix P. G., Malfant I.
Inorganic Chemistry 2023, 62 (49), 20349-20363.
https://doi.org/10.1021/acs.inorgchem.3c03355
https://hal.science/hal-04284209
Heteroleptic dirhodium(II) complexes with redox-active ferrocenyl ligands: Synthesis, electrochemical properties, and redox-responsive chemoselectivity in carbene C−H insertion
Ruzhylo I., Sournia-Saquet A., Moreau A., Delord T., Manoury E., Poli R., Labande A.
European Journal of Inorganic Chemistry 2022, 2022 (12), e202200033/1-10.
https://doi.org/10.1002/ejic.202200033
https://hal.archives-ouvertes.fr/hal-03528757
Redox-switchable behavior of transition-metal complexes supported by amino-decorated N-heterocyclic carbenes
Ruamps M., Bastin S., Rechignat L., Sournia-Saquet A., Vendier L., Lugan N., Mouesca J.-M., Valyaev D. A., Maurel V., César V.
Molecules 2022, 27 (12), 3776/1-17.
https://doi.org/10.3390/molecules27123776
https://hal.archives-ouvertes.fr/hal-03709012
Improving aqueous solubility and in vitro pharmacokinetic properties of the 3-nitroimidazo[1,2-a]pyridine antileishmanial pharmacophore
Paoli-Lombardo R., Primas N., Bourgeade-Delmas S., Hutter S., Sournia-Saquet A., Boudot C., Brenot E., Castera-Ducros C., Corvaisier S., Since M., Malzert-Fréon A., Courtioux B., Valentin A., Verhaeghe P., Azas N., Rathelot P., Vanelle P.
Pharmaceuticals 2022, 15 (8), 998/1-26.
https://doi.org/10.3390/ph15080998
https://hal.archives-ouvertes.fr/hal-03755519
Strong absorber vs. strong emitter in extended π-conjugated systems: a carbo-benzene – benzothiadiazole chromophore
Loaeza L., Maraval V., Saquet A., Ramos-Ortiz G., Chauvin R., Farfán N.
New Journal of Chemistry 2022, 46 (14), 6494-6501.
http://dx.doi.org/10.1039/D2NJ00569G
https://hal.archives-ouvertes.fr/hal-03631244
Carbo-mer of barrelene: a rigid 3D-carbon-expanded molecular barrel
Zhu C., Poater A., Duhayon C., Kauffmann B., Saquet A., Rives A., Maraval V., Chauvin R.
Chemistry – A European Journal 2021, 27 (36), 9286-9291.
https://doi.org/10.1002/chem.202100670
https://hal.archives-ouvertes.fr/hal-03228886v1
Oxidation-promoted synthesis of ferrocenyl planar chiral rhodium(III) complexes for C–H functionalization catalysis
Cabanes J., Odnoroh M., Duhayon C., Bijani C., Sournia-Saquet A., Polia R., Labande A.
Mendeleev Communications 2021, 31 (5), 620-623.
https://doi.org/10.1016/j.mencom.2021.09.010
https://hal.archives-ouvertes.fr/hal-03412564
New 8‑nitroquinolinone derivative displaying submicromolar in vitro activities against both Trypanosoma brucei and cruzi
Pedron J., Boudot C., Brossas J. Y., Pinault E., Bourgeade-Delmas S., Sournia-Saquet A., Boutet-Robinet E., Destere A., Tronnet A., Bergé J., Bonduelle C., Deraeve C., Pratviel G., Stigliani J. L., Paris L., Mazier D., Corvaisier S., Since M., Malzert-Fréon A., Wyllie S., Milne R., Fairlamb A. H., Valentin A., Courtioux B., Verhaeghe P.
ACS Medicinal Chemistry Letters 2020, 11, 464−472.
https://doi.org/10.1021/acsmedchemlett.9b00566
https://hal.archives-ouvertes.fr/hal-02476867
Antikinetoplastid SAR study in 3-nitroimidazopyridine series: Identification of a novel non-genotoxic and potent anti-T. b. brucei hit-compound with improved pharmacokinetic properties
Fersing C., Boudot C., Paoli-Lombardo R., Primas N., Pinault E., Hutter S., Castera-Ducros C., Kabri Y., Pedron J., Bourgeade-Delmas S., Sournia-Saquet A., Stigliani J.-L., Valentin A., Azqueta A., Muruzabal D., Destere A., Wyllie S., Fairlamb A. H., Corvaisier S., Since M., Malzert-Fréon A., Di Giorgio C., Rathelot P., Azas N., Courtioux B., Vanelle P., Verhaeghe P.
European Journal of Medicinal Chemistry 2020, 206, 112668/1-20.
https://doi.org/10.1016/j.ejmech.2020.112668
https://hal.archives-ouvertes.fr/hal-02920293
8-Alkynyl-3-nitroimidazopyridines display potent antitrypanosomal activity against both T. b. brucei and cruzi
Fersing C., Boudot C., Castera-Ducros C., Pinault E., Hutter S., Paoli-Lombardo R., Primas N., Pedron J., Seguy L., Bourgeade-Delmas S., Sournia-Saquet A., Stigliani J.-L., Brossas J.-Y., Paris L., Valentin A., Wyllie S., Fairlamb A. H., Boutet-Robinet É., Corvaisier S., Since M., Malzert-Fréon A., Destere A., Mazier D., Rathelot P., Courtioux B., Azas N., Verhaeghe P., Vanelle P.
European Journal of Medicinal Chemistry 2020, 202, 112558/1-15.
https://doi.org/10.1016/j.ejmech.2020.112558
https://hal.archives-ouvertes.fr/hal-02899751
On the spin-state dependence of redox potentials of spin crossover complexes
Dixon I. M., Rat S., Sournia-Saquet A., Molnár G., Salmon L., Bousseksou A.
Inorganic Chemistry 2020, 59 (24), 18402-18406.
https://doi.org/10.1021/acs.inorgchem.0c03043
https://hal.archives-ouvertes.fr/hal-03102204
Pentacyanoferrate(II) complex of pyridine-4- and pyrazine-2-hydroxamic acid as source of HNO: investigation of anti-tubercular and vasodilation activities
Carvalho E. M., de Freitas Paulo T., Sournia Saquet A., Abbadi B. L., Macchi F. S., Bizarro C. V., de Morais Campos R., Ferreira T. L. A., do Nascimento N. R. F., Lopes L. G. F., Chauvin R., Sousa E. H. S., Bernardes-Génisson V.
JBIC, Journal of Biological Inorganic Chemistry 2020, 25 (6), 887-901.
https://doi.org/10.1007/s00775-020-01805-z
https://hal.archives-ouvertes.fr/hal-02948991
Electrosynthesis of thin films of polythiophenes containing pyrene groups and flexible spacers, useful in the preparation of graphene polymer composites
Valderrama-García B. X., González-Méndez I., Sournia-Saquet A., Tassé M., Moineau-Chane Ching K. I., Rivera E.
MRS Advances 2019, 4 (59-60), 3233-3242.
https://doi.org/10.1557/adv.2019.410
https://hal.archives-ouvertes.fr/hal-02345035
Effect of solvent on silicon nanoparticle formation and size: a mechanistic study
Semlali S., Cormary B., De Marco M. L., Majimel J., Saquet A., Coppel Y., Gonidec M., Rosa P., Drisko G. L.
Nanoscale 2019, 11 (11), 4696-4700.
http://dx.doi.org/10.1039/C9NR00619B
https://hal.archives-ouvertes.fr/hal-02071674
Further studies on the photoreactivities of ruthenium–nitrosyl complexes with terpyridyl ligands
Sasaki I., Amabilino S., Mallet-Ladeira S., Tassé M., Sournia-Saquet A., Lacroix P. G., Malfant I.
New Journal of Chemistry 2019, 43 (28), 11241-11250.
http://dx.doi.org/10.1039/C9NJ02398D
https://hal.archives-ouvertes.fr/hal-02321845
Dinuclear copper(I) complexes combining bis(diphenylphosphanyl)acetylene with 1,10-phenanthroline ligands
Nierengarten J.-F., Nierengarten I., Holler M., Sournia-Saquet A., Delavaux-Nicot B., Leoni E., Monti F., Armaroli N.
European Journal of Inorganic Chemistry 2019, (22), 2665-2673.
https://doi.org/10.1002/ejic.201900335
https://hal.archives-ouvertes.fr/hal-02335003
Nongenotoxic 3-Nitroimidazo[1,2-a]pyridines are NTR1 substrates that display potent in vitro antileishmanial activity
Fersing C., Basmaciyan L., Boudot C., Pedron J., Hutter S., Cohen A., Castera-Ducros C., Primas N., Laget M., Casanova M., Bourgeade-Delmas S., Piednoel M., Sournia-Saquet A., Belle Mbou V., Courtioux B., Boutet-Robinet E., Since M., Milne R., Wyllie S., Fairlamb A. H., Valentin A., Rathelot P., Verhaeghe P., Vanelle P., Azas N.
ACS Medicinal Chemistry Letters 2019, 10 (1), 34-39.
https://doi.org/10.1021/acsmedchemlett.8b00347
https://hal.archives-ouvertes.fr/hal-02478935
Carbo-biphenyls and carbo-terphenyls: Oligo(phenylene ethynylene) ring carbo-mers
Zhu C., Poater A., Duhayon C., Kauffmann B., Saquet A., Maraval V., Chauvin R.
Angewandte Chemie, International Edition 2018, 57 (20), 5640-5644.
http://doi.org/10.1002/anie.201713411
https://hal.archives-ouvertes.fr/hal-01952873
Unveiling the redox-active character of imidazolin-2-thiones derived from amino-substituted N-heterocyclic carbenes
Ruamps M., Bastin S., Rechignat L., Sournia-Saquet A., Valyaev D. A., Mouesca J.-M., Lugan N., Maurel V., Cesar V.
Chemical Communications 2018, 54 (55), 7653-7656.
http://dx.doi.org/10.1039/C8CC03934H
https://hal.archives-ouvertes.fr/hal-01948412
Efficient analoging around ethionamide to explore thioamides bioactivation pathways triggered by boosters in Mycobacterium tuberculosis
Prieri M., Frita R., Probst N., Sournia-Saquet A., Bourotte M., Deprez B., Baulard A. R., Willand N.
European Journal of Medicinal Chemistry 2018, 159, 35-46.
https://doi.org/10.1016/j.ejmech.2018.09.038
https://hal.archives-ouvertes.fr/hal-01963620
Novel 8-nitroquinolin-2(1H)-ones as NTR-bioactivated antikinetoplastid molecules: Synthesis, electrochemical and SAR study
Pedron J., Boudot C., Hutter S., Bourgeade-Delmas S., Stigliani J.-L., Sournia-Saquet A., Moreau A., Boutet-Robinet E., Paloque L., Mothes E., Laget M., Vendier L., Pratviel G., Wyllie S., Fairlamb A., Azas N., Courtioux B., Valentin A., Verhaeghe P.
European Journal of Medicinal Chemistry 2018, 155, 135-152.
https://doi.org/10.1016/j.ejmech.2018.06.001
https://hal.archives-ouvertes.fr/hal-01888465
Antitrypanosomatid pharmacomodulation at position 3 of the 8-nitroquinolin-2(1H)-one scaffold using palladium-catalysed cross-coupling reactions
Pedron J., Boudot C., Bourgeade-Delmas S., Sournia-Saquet A., Paloque L., Rastegari M., Abdoulaye M., El-Kashef H., Bonduelle C., Pratviel G., Wyllie S., Fairlamb A. H., Courtioux B., Verhaeghe P., Valentin A.
ChemMedChem 2018, 13 (20), 2217-2228.
https://doi.org/10.1002/cmdc.201800456
https://hal.archives-ouvertes.fr/hal-01888530
Steric/π-electronic insulation of the carbo-benzene ring: Dramatic effects of tert-butyl versus phenyl crowns on geometric, chromophoric, redox, and magnetic properties
Listunov D., Duhayon C., Poater A., Mazeres S., Saquet A., Maraval V., Chauvin R.
Chemistry – A European Journal 2018, 24 (42), 10699-10710.
http://doi.org/10.1002/chem.201800835
https://hal.archives-ouvertes.fr/hal-01952878
Heteroleptic copper(I) complexes prepared from phenanthroline and bis-phosphine ligands: Rationalization of the photophysical and electrochemical properties
Leoni E., Mohanraj J., Holler M., Mohankumar M., Nierengarten I., Monti F., Sournia-Saquet A., Delavaux-Nicot B., Nierengarten J.-F., Armaroli N.
Inorganic Chemistry 2018, 57 (24), 15537-15549.
https://doi.org/10.1021/acs.inorgchem.8b02879
https://hal.archives-ouvertes.fr/hal-02129895
In situ metalorganic deposition of silver nanoparticles on gold substrate and square wave voltammetry: A highly efficient combination for nanomolar detection of nitrate ions in sea water
Lebon E., Fau P., Comtat M., Kahn M. L., Sournia-Saquet A., Temple-Boyer P., Dubreuil B., Behra P., Fajerwerg K.
Chemosensors 2018, 6 (4), 50/1-12.
https://doi.org/10.3390/chemosensors6040050
https://hal.archives-ouvertes.fr/hal-02336687
In situ metalorganic deposition of silver nanoparticles on gold substrate and square wave voltammetry: A highly efficient combination for nanomolar detection of nitrate ions in sea water
Lebon E., Fau P., Comtat M., Kahn M. L., Sournia-Saquet A., Temple-Boyer P., Dubreuil B., Behra P., Fajerwerg K.
Chemosensors 2018, 6 (4), 50/1-12.
https://doi.org/10.3390/chemosensors6040050
https://hal.archives-ouvertes.fr/hal-02336687
Synthesis and mechanistic investigation of iron(II) complexes of isoniazid and derivatives as a redox-mediated activation strategy for anti-tuberculosis therapy
Laborde J., Deraeve C., de Mesquita Vieira F. G., Sournia-Saquet A., Rechignat L., Drumond Villela A., Lopes Abbadi B., Souza Macchi F., Pissinati K., Bizarro C. V., Machado P., Augusto Basso L., Pratviel G., Gonzaga de França Lopes L., Silva Sousa E. H., Bernardes-Genisson V.
Journal of Inorganic Biochemistry 2018, 179, 71-81.
https://doi.org/10.1016/j.jinorgbio.2017.11.013
https://hal.archives-ouvertes.fr/hal-01952877
8-Aryl-6-chloro-3-nitro-2-(phenylsulfonylmethyl)imidazo[1,2-a]pyridines as potent antitrypanosomatid molecules bioactivated by type 1 nitroreductases
Fersing C., Boudot C., Pedron J., Hutter S., Primas N., Castera-Ducros C., Bourgeade-Delmas S., Sournia-Saquet A., Moreau A., Cohen A., Stigliani J.-L., Pratviel G., Crozet M. D., Wyllie S., Fairlamb A., Valentin A., Rathelot P., Azas N., Courtioux B., Verhaeghe P., Vanelle P.
European Journal of Medicinal Chemistry 2018, 157, 115-126.
https://doi.org/10.1016/j.ejmech.2018.07.064
https://hal.archives-ouvertes.fr/hal-01909649
A novel method for the metallization of 3D silicon induced by metastable copper nanoparticles
Cure J., Piettre K., Sournia-Saquet A., Coppel Y., Esvan J., Chaudret B., Fau P.
Acs Applied Materials & Interfaces 2018, 10 (38), 32838-32848.
https://doi.org/10.1021/acsami.8b09428
https://hal.archives-ouvertes.fr/hal-01962590
Homo- and heteropolymetallic complexes of the hybrid, ambidentate N-heterocyclic carbene Ligand IMes-acac
Cesar V., Mallardo V., Nano A., DePeter S. F., Bastin S., Sournia-Saquet A., Maisse-François A., Lugan N., Bellemin-Laponnaz S.
Acs Omega 2018, 3 (11), 15582-15591.
https://doi.org/10.1021/acsomega.8b02268
https://hal.archives-ouvertes.fr/hal-01948413
Selective access to p-dialkyl-carbo-benzenes from a [6]pericyclynedione: the n-butyl nucleophile model for a metal switch study
Zhu C. W., Duhayon C., Saquet A., Maraval V., Chauvin R.
Canadian Journal of Chemistry 2017, 95 (4), 454-459.
http://dx.doi.org/10.1139/cjc-2016-0629
https://hal.science/hal-01939390v1
Hexaaryl-carbo-benzenes revisited: a novel synthetic route, crystallographic data, and prospects of electrochemical behavior
Zhu C. W., Duhayon C., Romero-Borja D., Maldonado J. L., Ramos-Ortiz G., Saquet A., Maraval V., Chauvin R.
New Journal of Chemistry 2017, 41 (10), 3908-3914.
http://dx.doi.org/10.1039/c7nj00028f
https://hal.science/hal-01939389v1
CH bond activation of unsaturated hydrocarbons by a niobium methyl cyclopropyl precursor. Cyclopropyl ring opening and alkyne coupling reaction
Oulié P., Dinoi C., Li C., Sournia-Saquet A., Jacob K., Vendier L., Etienne M.
Organometallics 2017, 36 (1), 53-63.
http://dx.doi.org/10.1021/acs.organomet.6b00506
https://hal.science/hal-01939584v1
Synthesis, oxidation potential and anti–mycobacterial activity of isoniazid and analogues: Insights into the molecular isoniazid activation mechanism
Laborde J., Deraeve C., Lecoq L., Sournia-Saquet A., Stigliani J.-L., Orena B. S., Mori G., Pratviel G., Bernardes-Genisson V.
ChemistrySelect 2016, 1 (2), 172-179.
http://dx.doi.org/10.1002/slct.201600040
https://hal.science/hal-01929903v1
Ordered layered dendrimers constructed from two known dendrimer families: Inheritance and emergence of properties
Dib H., Rebout C., Laurent R., Mallet-Ladeira S., Sournia-Saquet A., Sarosi M. B., Hey-Hawkins E., Majoral J.-P., Delavaux-Nicot B., Caminade A.-M.
Chemistry – A European Journal 2016, 22 (31), 10736-10742.
http://dx.doi.org/10.1002/chem.201601354
https://hal.archives-ouvertes.fr/hal-01933090v1
Carbo-cyclohexadienes vs. carbo-benzenes: Structure and conjugative properties
Rives A., Baglai I., Barthes C., Maraval V., Saffon-Merceron N., Saquet A., Voitenko Z., Volovenko Y., Chauvin R.
Chemical Science 2015, 6 (2), 1139-1149.
http://dx.doi.org/10.1039/C4SC02742F
https://hal.science/hal-01915500v1
Ruthenium complexes with dendritic ferrocenyl phosphanes: Synthesis, characterization, and application in the catalytic redox isomerization of allylic alcohols
Neumann P., Dib H., Sournia-Saquet A., Grell T., Handke M., Caminade A.-M., Hey-Hawkins E.
Chemistry – A European Journal 2015, 21 (17), 6590-6604.
http://dx.doi.org/10.1002/chem.201406489
https://hal.science/hal-01923213v1
Electron transfer rates in an adsorbed C-60-porphyrin dyad
Fortgang P., Urbani M., Holler M., Nierengarten J.-F., Moreau A., Delavaux-Nicot B., Maisonhaute E.
Electroanalysis 2015, 27 (4), 1010-1016.
http://dx.doi.org/10.1002/elan.201400618
https://hal.archives-ouvertes.fr/hal-01298208v1
Carbo-quinoids: Stability and reversible redox- proaromatic character towards carbo-benzenes
Cocq K., Maraval V., Saffon-Merceron N., Saquet A., Poidevin C., Lepetit C., Chauvin R.
Angewandte Chemie, International Edition 2015, 54 (9), 2703-2706.
http://dx.doi.org/10.1002/anie.201407889
https://hal.science/hal-01915507v1</a
Heteroleptic bis(cis-1,2-disubstituted ethylene-1,2-dithiolato)nickel complexes obtained by ligand-exchange reaction: Synthesis and properties
Vuong T. M. H., Bui T.-T., Sournia-Saquet A., Moreau A., Moineau-Chane Ching K. I.
Inorganic Chemistry 2014, 53 (6), 2841-2847.
http://dx.doi.org/10.1021/ic402528j
https://hal.science/hal-00992334v1
Characterization of new specific copper chelators as potential drugs for the treatment of Alzheimer’s disease
Nguyen M., Robert A., Sournia-Saquet A., Vendier L., Meunier B.
Chemistry – A European Journal 2014, 20 (22), 6771-6785.
http://dx.doi.org/10.1002/chem.201402143
https://hal.science/hal-02023147v1
Chain ordering of regioregular polythiophene films through blending with a nickel bisdithiolene complex
Hernandez-Maldonado D., Ramos B., Villeneuve-Faure C., Bedel-Pereira E., Seguy I., Sournia-Saquet A., Alary F., Heully J. L., Moineau-Chane Ching K. I.
Applied Physics Letters 2014, 104 (10), 103302/1-4.
http://dx.doi.org/10.1063/1.4868106
https://hal.science/hal-00984302v1
Oxidation-promoted activation of a ferrocene C-H bond by a rhodium complex
Labande A., Debono N., Sournia-Saquet A., Daran J.-C., Poli R.
Dalton Transactions 2013, 42 (18), 6531-6537.
http://dx.doi.org/10.1039/C3DT50240F
https://hal.archives-ouvertes.fr/hal-02908070
Synthesis and characterization of water-soluble ferrocene-dendrimers
de Jong E. R., Manoury E., Daran J.-C., Turrin C. O., Chiffre J., Sournia-Saquet A., Knoll W., Majoral J.-P., Caminade A.-M.
Journal of Organometallic Chemistry 2012, 718, 22-30.
http://dx.doi.org/10.1016/j.jorganchem.2012.07.048
https://hal.science/hal-02909731v1
Synthesis, X-ray crystal structures, optical properties and modelling data of neutral bis(1,2-dithiolene) nickel complexes of the “non-cyclic SR” family
Bui T. T., Vuong M. H., Garreau-de Bonneval B., Alary F., Kane J., Duhayon C., Sournia-Saquet A., Moineau-Chane Ching K. I.
New Journal of Chemistry 2012, 36 (10), 2033-2041.
http://dx.doi.org/10.1039/c2nj40398f
https://hal.science/hal-00834748v1
Tuning of the emission efficiency and HOMO–LUMO band gap for ester-functionalized {Al(salophen)(H2O)2}+ blue luminophors
Béreau V., Duhayon C., Sournia-Saquet A., Sutter J.-P.
Inorganic Chemistry 2012, 51 (3), 1309-1318.
http://dx.doi.org/10.1021/ic201208c
https://hal.science/hal-02909324v1
1,4-Dialkynylbutatrienes: synthesis, stability, and perspectives in the chemistry of carbo-benzenes
Maraval V., Leroyer L., Harano A., Barthes C., Saquet A., Duhayon C., Shinmyozu T., Chauvin R.
Chemistry – A European Journal 2011, 17 (18), 5085-5099.
http://dx.doi.org/10.1002/chem.201002769
Synthesis and photophysical properties of copper(I) complexes obtained from 1,10-phenanthroline ligands with increasingly bulky 2,9-substituents
Accorsi G., Armaroli N., Duhayon C., Saquet A., Delavaux-Nicot B., Welter R., Moudam O., Holler M., Nierengarten J. F.
European Journal of Inorganic Chemistry 2010, 2010 (1), 164-173.
http://dx.doi.org/10.1002/ejic.200900954
https://hal.archives-ouvertes.fr/hal-02908203v1
Dinuclear gold(I) and gold(III) complexes of bridging functionalized bis(N-heterocyclic carbene) ligands: synthesis, structural, spectroscopic and electrochemical characterizations
Jean-Baptiste dit Dominique F., Gornitzka H., Sournia-Saquet A., Hemmert C.
Dalton Transactions 2009, 38 (2), 340-352.
http://dx.doi.org/10.1039/B809943J
Fullerene derivatives functionalized with diethylamino-substituted conjugated oligomers: synthesis and photoinduced electron transfer
Gégout A., Nierengarten J.-F., Delavaux-Nicot B., Duhayon C., Saquet A., Listorti A., Belbakra A., Chiorboli C., Armaroli N.
Chemistry – A European Journal 2009, 15 (35), 8825-8833.
http://dx.doi.org/10.1002/chem.200901216
https://hal.archives-ouvertes.fr/hal-03839518
Electrochemical properties and electronic structures of two neutral nickel bis(1,2-dithiolene) complexes
Sournia-Saquet A., Garreau de Bonneval B., Chane-Ching K. I., Valade L.
Journal of Electroanalytical Chemistry 2008, 624, 84-90.
https://doi.org/10.1016/j.jelechem.2008.08.001
Organotin chemistry for the preparation of fullerene-rich nanostructures
Delavaux-Nicot B., Kaeser A., Hahn U., Gégout A., Brandli P.-E., Duhayon C., Coppel Y., Saquet A., Nierengarten J.-F.
Journal of Materials Chemistry 2008, 18 (13), 1547-1554.
https://doi.org/10.1039/B716506D
https://hal.archives-ouvertes.fr/hal-03839310
Synthesis, spectroscopic, structural and electrochemical studies of carboxyl substituted 1,4-naphthoquinones
Boudalis A. K., Policand X., Sournia-Saquet A., Donnadieu B., Tuchagues J.-P.
Inorganica Chimica Acta 2008, 361, 1681-1688.
https://doi.org/10.1016/j.jelechem.2008.08.001
Proton reduction catalysis by manganese vinylidene and allenylidene complexes
Valyaev D. A., Peterleitner M. G., Semeikin O. V., Utegenov K. I., Ustynyuk N. A., Sournia-Saquet A., Lugan N., Lavigne G.
Journal of Organometallic Chemistry 2007, 692, 3207-3211.
https://doi.org/10.1016/j.jorganchem.2007.01.055
https://hal.science/hal-03739916v1
Quadratic nonlinear optical response in partially charged donor-substitued tetrathiafulvalene : from a computational investigation to a rational synthetic feasibility
Lamere J.-F., Malfant I., Sournia-Saquet A., Lacroix P. G., Fabre J.-M., Kaboub L., Abbaz T., Krim Gouasmia A., Asselberghs I., Clays K.
Chemistry of Materials 2007, 19, 805-815.
https://doi.org/10.1021/cm0623110
Self-assembly of fullerene-rich nanostructures with a stannoxane core
Hahn U., Gégout A., Duhayon C., Coppel Y., Sournia-Saquet A., Nierengarten J.-F.
Chemical Communications 2007, 5, 516-518.
https://doi.org/10.1039/B614009B
Redox chemistry of copper-amyloid-ß : the generation of hydroxyl radical in the presence of ascorbate is linked to redox-potentials and aggregation state
Guilloreau L., Combalbert S., Sournia-Saquet A., Mazarguil H., Faller P.
ChemBioChem 2007, 8, 1317-1325.
https://doi.org/10.1002/cbic.200700111
Facile synthesis of cyclometalated ruthenium complexes with substituted phenylpyridines
Sasaki I., Vendier L., Sournia-Saquet A., Lacroix P. G.
European Journal of Inorganic Chemistry 2006, 16, 3294-3302.
https://doi.org/10.1002/ejic.200600359
From calcium interaction to calcium electrochemial detection by [(C5H5)Fe(C5H4COCH=CHC6H4NEt2)] and its two novel structurally characterized derivatives
Maynadié J., Delavaux-Nicot B., Lavabre D., Donnadieu B., Daran J.-C., Sournia-Saquet A.
Inorganic Chemistry 2004, 43 (6), 2064-2077.
https://doi.org/10.1021/ic0345828
https://hal.archives-ouvertes.fr/hal-03839057
Electrocatalytic dimerisation of non-heteroatom-substituted manganese alkynylcarbene complexes
Ortin Y., Sournia-Saquet A., Lugan N., Mathieu R.
Chemical Communications 2003, 1060-1061.
https://doi.org/10.1039/B300623A
LCC
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




