LCC
Molecular Complexes for Energy Transition
Hydrogen Storage and Release
Our fundamental research activity focuses on the molecular chemistry of transition metals and the chemistry of heteroelements from groups 13 to 15, such as phosphorus, boron, and silicon. Our contribution to the energy transition aims to leverage our expertise to address current challenges in the development of renewable energies.
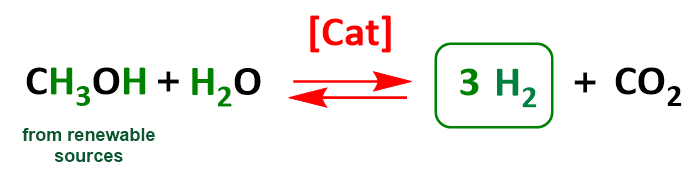
Our work involves the design, synthesis, and evaluation of molecular complexes for catalytic hydrogenation and dehydrogenation reactions. The targeted goal is the storage and release of hydrogen via an organic liquid carrier (formic acid, methanol) for potential large-scale industrial applications.
Example 1 :
– Shvo-Type Metal−Ligand Cooperative Catalysts: Tethered η5‑Oxocyclohexadienyl Ruthenium Complexes, E. Puig, R. Verron, M. Kechaou-Perrot, L. Vendier, H. Gornitzka, K. Miqueu, J.-M. Sotiropoulos, C. Fischmeister, P. Sutra, A. Igau, Organometallics 2022, 41 (11), 1391-1402 doi.org/10.1021/acs.organomet.2c00123
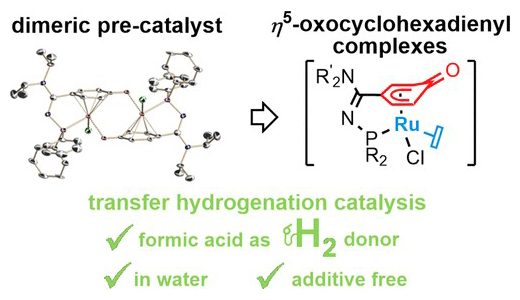
Example 2 :
– Base-Free Reversible Hydrogen Storage Using a Tethered p‑Coordinated-Phenoxy Ruthenium-Dimer Precatalyst, R. Verron, E. Puig, P. Sutra, A. Igau, C. Fischmeister ACS Catal. 2023, 13, 5787−5794 https://doi.org/10.1021/acscatal.3c00476 ;
As part of the SinCare-H₂ project (Single Catalyst for Reversible Hydrogen Storage and Release “in/from” Methanol Carrier), we have developed a single catalyst capable of performing several reversible hydrogen storage cycles via formic acid without any additive.
This catalyst exhibits a latency effect, allowing the preparation of a ready-to-use formic acid solution for hydrogen release. This concept is an ideal concept for the transport and delivery of hydrogen to end-use devices.
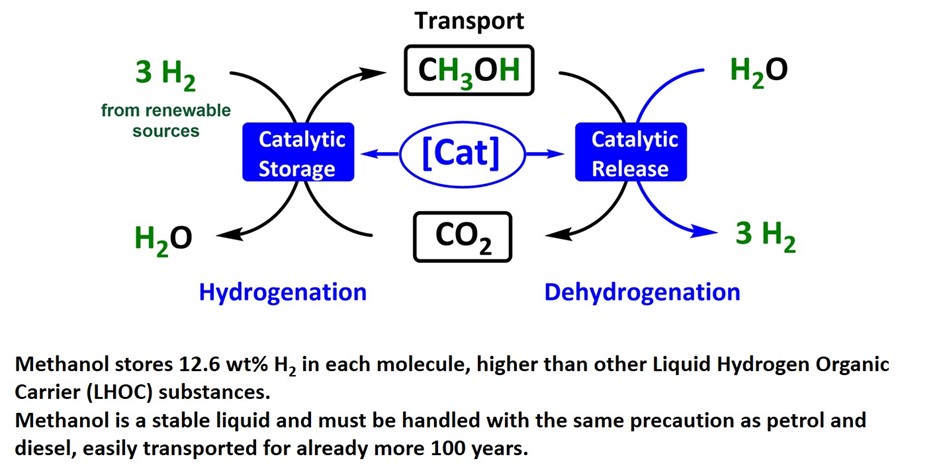
Exemple 3 :
Another project focuses on the use of methanol as a liquid hydrogen reservoir:
Collaborations on this topic include:
- In Rennes: Cédric Fischmeister, Institut des Sciences Chimiques de Rennes, UMR 6226, Université de Rennes
- In Pau: Jean-Marc Sotiropoulos, Philippe Carbonnière, CNRS/Université de Pau et des Pays de l’Adour, Institut des Sciences Analytiques et de Physico-Chimie pour l’Environnement et les Matériaux, UMR CNRS 525
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




