LCC
Since the 2000’s a lot of regulations for the chemical and pharmaceutical industries have appeared especially in terms of efficiency, waste management and energy input. All these issues are now addressed and termed « Green Chemistry » a multifaceted field dealing with what we call the twelve principles of P. T. Anastas and J. C. Warner. Most important of them are: atom economy, preventing the use of solvents volatile and/or toxic, minimize chemical waste and minimize energy. By focusing on the Green Chemistry reactions, the alternative energy sources that appeared and developed are: photochemistry through light excitation, microwave, sonochemistry irradiation, and mechanochemistry.
According to IUPAC, a mechanochemical reaction is a “Chemical reaction that is induced by the direct absorption of mechanical energy”. Wilhelm Ostwald (Nobel Prize in 1909), was the first who mentioned the term “Mechanochemistry” and defined it as a “branch of chemistry which is concerned with chemical and physico-chemical changes of substances of all states of aggregation due to the influence of mechanical energy”. In the two last decades, mechanochemistry has been developed considerably in a multitude of areas such as: inorganic compounds and metal complexes synthesis, catalysis, polymers, nanomaterials, and organic synthesis used for creating carbon-carbon, carbon-heteroatom, metal-ligand coordination bonds. It is important to point out also the many efforts developed towards mechanistic level understanding of mechanochemical processes and the possible links between the mechanical effect and the action of the forces generated at the molecular level.
The “mechanochemistry programme” of the team has been initiated in 2012 and developed two important aspects: (a) fundamental mechanistic studies on selected reactions and (b) medicinal mechanochemistry. The projects and research programs in the mechanochemistry field (but also green chemistry approaches through micro-wave activation) that we are currently focused concern:
- i) multicomponent reactions namely, Biginelli and domino reactions
- ii) synthesis of hydrazones, chalcones, 1,2,4-triazoles and functionalized terpyridines as important families of compounds per se but also through complexation
- iii) complexation studies of the ligands with transition metals, namely ruthenium and RuNO complexes
- iv) theoretical and spectro-physical studies of all complexes elaborated, especially kinetics of NO photo-release
- v) Evaluation of their biological activities and studies of their mechanism of action (antitumoral, antibacterial, antiparasitary)
- vi) coupling to nanocarriers for potential applications in bioconjugation and targeted drug delivery.
The two first aspects have been already successfully conducted. Especially, in the near future hydrazones and functionalized terpyridines will be used as ligands for the elaboration of Ruthenium and Ru-NO complexes. This research is part of a European project (compounds with antiparasitary activities) obtained in October 2025 (POCTEFA) that the team coordinates.
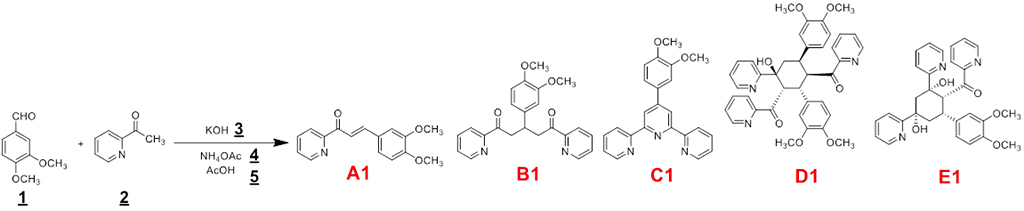
Example of a mechanochemical reaction leading to a 2,2’:6,2’’-terpyridine (C1)
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




