LCC
The use of RuNO complexes in biological tissues raises the delicate question of their form (molecules dispersed in solution, grafted onto nanoparticles, dendrimers or polymers, inserted into micellar structures, etc.). Obtaining these objects offers several advantages because, unlike isolated molecules in solution that can only penetrate cells by diffusion, these objects can be captured by cells through much more efficient mechanisms (e.g., phagocytosis), which lead to a double effect:
- increased penetration of NO carriers,
- photo-release of multiple NO units present in the carrier particle.
Nanoparticles of ruthenium nitrosyl
(Chem. Phys. Lett., 2023, 818, 140434)
We have shown that it is possible to obtain nanoscale aggregates of the trans(Cl,Cl)-[RuFTCl2NO]PF6 complex by controlled precipitation in aqueous solution (Figure 1). The average diameter of the nanoparticles depends on experimental conditions, in particular the rate at which the RuNO complex solution is added to the aqueous phase. The quantum yield of photorelease (ΦNO) of the nano-aggregates is around 0.12 (irradiation at 365 or 400 nm). It is independent of their size and state of dispersion. The quantum yield of the nanoparticles is very similar to that of the same complex as isolated molecules in solution. These molecular nano-objects can therefore be considered as a nano-platform for NO release for antibacterial activity.
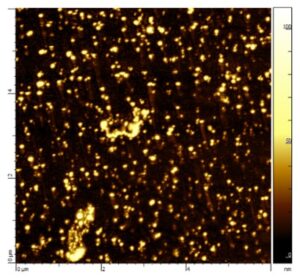
Figure 1: AFM image of nanoparticles of the trans(Cl,Cl)-[RuFTCl2NO]PF6 complex in water (mean diameter: 16 nm)
Ruthenium nitrosyl complexes embedded in hydrogels
(New J. Chem., 2024, 48, 8343)
A hydrogel is a gel in which the swelling agent is water. Hydrogels derived from Pluronic F127 (PL) are capable of spontaneously forming micelles in water. Their organization leads to the formation of a gel in the 20-40°C range, which is compatible with medical applications. Although perfectly biocompatible and non-toxic, they suffer from poor mechanical strength. This can be greatly improved by adding chitosan (CS), which promotes cross-linking. We used these PL/CS hydrogels as a support for incorporating a RuNO complex.
We incorporated the water-soluble trans(Cl,Cl)-[RuFTCl2NO]Cl complex into PL/CS hydrogels at RuNO concentrations ranging from 13 to 520 µg.g‒1 without observing any demixing (Figure 2). The hydrogels show a sol-gel transition between 35 and 40°C depending on the RuNO complex content (35°C for the hydrogel without RuNO). It is therefore little affected by the incorporation and concentration of the complex, which makes it suitable for cutaneous applications. Under irradiation at 400 nm and at physiological temperature (37°C), PL/CS/RuNO hybrid hydrogels are capable of releasing nitric oxide, as demonstrated by electron paramagnetic resonance (Figure 3) and electrochemical detection (NO- sensor). The quantum yield of of photorelease (ΦNO) is 0.014 (at 37°C for irradiation at 400 nm).
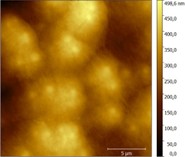
Figure 2: AFM image of a PL/CS hydrogel incorporating the trans(Cl,Cl)-[RuFTCl2NO]Cl complex (13 µg.g‒1)
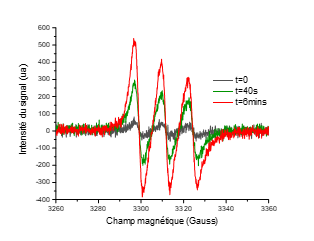
Figure 3: EPR spectra for a PL/CS hydrogel incorporating the trans(Cl,Cl)-[RuFTCl2NO]Cl complex (13 µg.g‒1)
Platinum nanoparticles functionalized by a ruthenium nitrosyl complex
We have prepared the first metal nanoparticles functionalized with a ruthenium nitrosyl derivative. Platinum nanoparticles with the general formula Ptx(CO)y(CH3CN)z, synthesized by Simon Tricard’s team at the LPCNO in Toulouse, were dispersed in acetonitrile and mixed with the trans(Cl,Cl)-[RuTTCl2NO]PF6 complex (Figure 4).
The substitution of some CH3CN and/or CO ligands by the thiophene group of the terpyridine ligand of the RuNO complex was evidenced by infrared spectroscopy. The functionalized platinum nanoparticles, with the postulated formula Ptx’(CO)y’(CH3CN)z’(RuNO)t, form aggregates in which the size of the individual particles is 1 to 3 nm (Figure 5).
Current-voltage characteristics of the nanoparticles show greater Coulomb blocking than PPtx(CO)y(CH3CN)z, consistent with the introduction of an additional molecular barrier at the interface and a reduction in electronic coupling between adjacent nanoparticles. Finally, EPR evidences the photo-release of NO under 365 nm irradiation for Ptx’(CO)y’(CH3CN)z’(RuNO)t nano-objects. We are currently studying the possible influence of plasmon excitation on the efficiency of nitrogen monoxide release in solution.
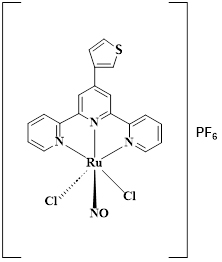
Figure 4 : Molecular formula for trans(Cl,Cl)-[RuTTCl2NO]PF6
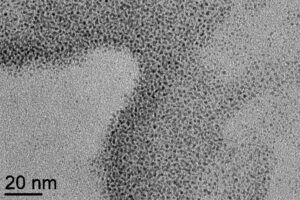
Figure 5 : TEM image of platinum nanoparticles functionalized by the trans(Cl,Cl)-[RuTTCl2NO]PF6
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




