LCC
In situ/operando spectroscopy
We use various spectroscopic techniques to observe the changes that occur during the reaction. We implement reaction conditions that are as close as possible to those used in the laboratory.
We use low-energy X-rays to identify phase/crystallinity changes during the reaction, high-energy X-rays at the synchrotron to identify changes in oxidation state and coordination, Fourier transform infrared spectroscopy to identify functional groups, and microscopy to determine metal dispersion/agglomeration.
Reference
Operando spectroscopy to understand dynamic structural changes of solid catalysts
Sarma B. B., Grunwaldt J.-D.
CHIMIA 2024, 78(5), 288-296.
https://doi.org/10.2533/chimia.2024.288
https://hal.science/hal-04593870
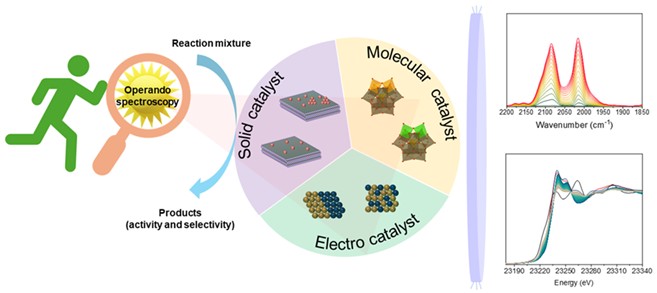
Fig. Various in situ/operando techniques for monitoring dynamic changes in the catalyst
Dynamic behaviour of single atom catalysts and clusters
Solid catalysts exhibit highly dynamic behaviour and tend to restructure during the reaction. Among them, single atom catalysts (SACs) or atomically dispersed catalysts are more vulnerable when the dynamic behaviour of catalysts plays a key role. SACs have the potential to achieve maximum efficiency (theoretically), but on the other hand, due to their high surface energy, they tend to agglomerate, which alters the reaction pathway. It is therefore difficult to know whether the active site is an isolated metal or a metal island. To understand dynamic behaviour, in situ/operando spectroscopy is essential. For example, in our recent work, we have shown that Rh SACs during ethylene hydroformylation form Rh clusters, leading to two different pathways (hydroformylation reaction and CO hydrogenation).
Reference
Understanding the role of supported Rh atoms and clusters during hydroformylation and CO hydrogenation reactions with in situ/operando XAS and DRIFT spectroscopy
Sarma B. B., Neukum D., Doronkin D. E., Lakshmi Nilayam A. R., Baumgarten L., Krause B., Grunwaldt J.-D.
Chemical Science 2024, 15(31), 12369-12379.
http://dx.doi.org/10.1039/D4SC02907K
https://hal.science/hal-04692606
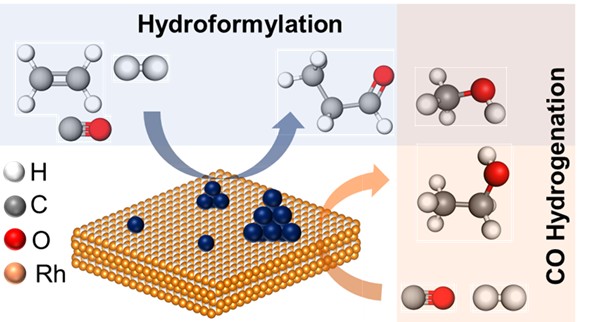
Fig. Dynamic behaviour of the single-atom rhodium catalyst during the hydroformylation reaction. Credit : Bidyut B. Sarma
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




