LCC
Supramolecular microporous architectures
We explore an original approach towards microporous architectures using ionic hydrogen bonding as linkage for 3D opened-structures. These architectures, named SPA, are prepared under mild conditions in aqueous medium using H-bond donor, typically protonated poly-pyridinic molecules, and anionic metal-oxalate complex as acceptor of H-bonds (illustration).
Robustness of ionic H-bond allows modification of R function of the organic part while keeping same supramolecular assembly.
In this way, the channels walls are covered by chemical functions modulating characteristics and sorption affinities of the materials. For example, SPA-2 functionalized with pyridine groups (blue spheres in illustration) can trap acidic molecules.[1] Same approach can be applied to tune water sorption characteristics [2] or to capture of noble metal ions.[3]
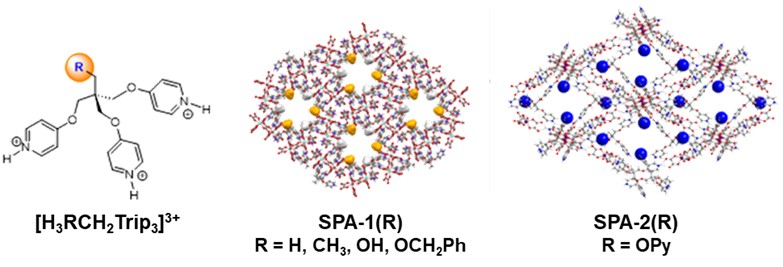
Illustration: Tri-pyridinic derivatives and opened architectures SPA-1(R) and SPA-2(OPy) obtained with association of [Al(oxalate)3]3- and [Zr2(oxalate)7]6-, respectively.
Recent works:
[1] Hydrogen-Bonded Open-Framework with Pyridyl-Decorated Channels: Straightforward Preparation and Insight into its Affinity for Acidic Molecules in Solution.
G. Mouchaham, N. Roques, W. Khodja, C. Duhayon, Y. Coppel, S. Brandès, T. Fodor, M. Meyer, J.-P. Sutter.
Chem. Eur. J., 2017, 23, 11818-11826.
http://dx.doi.org/10.1002/chem.201701732
[2] Modulation of the sorption characteristics for an H-bonded porous architecture by varying the chemical functionalization of the channel walls.
Roques, A. Tovar-Molle, C. Duhayon, S. Brandès, A. Spieß, C. Janiak, J.-P. Sutter.
Chem. Eur. J., 2022, 28, 61, e202201935/1-10.
https://doi.org/10.1002/chem.202201935
[3] Controlled Growth of Ag NanoCrystals in a H-bonded Open-Framework.
W. Khodja, V. Collière, M. L. Kahn, N. Roques, J.-P. Sutter.
Chem. Eur. J., 2019, 25, 13705-13708.
https://doi.org/10.1002/chem.201903684
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




