
- This event has passed.
Seminar (2) Pr Evamarie HEY-HAWKINS

Better Together! Phosphorus Meets Carborane
Prof. Dr. Evamarie Hey-Hawkins
Leipzig University, Germany
Abstract
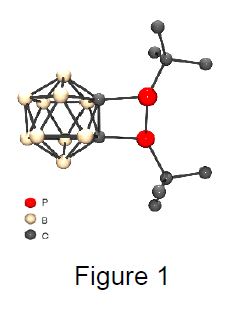
Phosphorus Icosahedral dicarba-closo-dodecaboranes (carboranes) provide an excellent scaffold for phosphines due to their unusual steric (bulky, flexible C-C bond in ortho-carborane) and electronic properties. Thus, the carboranyl backbone allows the targeted synthesis of four- and five-membered phosphorus-containing heterocycles featuring endocyclic P–P bonds, which are difficult to obtain via other routes. 1,2-Bisphosphanyl-substituted ortho-carboranes, the precursors for 1,2-diphosphetanes (Figure 1), 1,2,3-triphospholanes, 1,2,3-triphospholanides, and other carboranylsubstituted heterocycles will be discussed.[1]
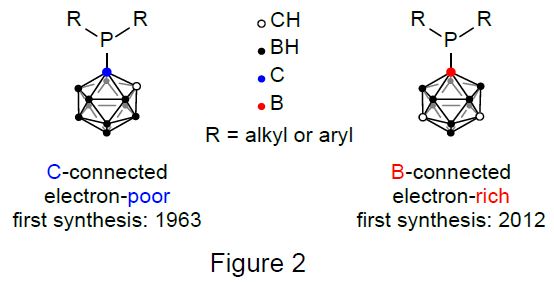
Furthermore, phosphines containing Ccarboranyl substituents tend to be electron-poor, while B9-connected carboranylphosphines have in fact shown to be electron-rich (Figure 2), surpassing the donor strength of alkyl phosphines and even commonly used N-heterocyclic carbenes.[2]
In this presentation, the influence of the carboranyl backbone in ring-opening reactions, redox reactions, including formation of phosphoniumyl radical cations, and selected examples of coordination chemistry will be covered.[2,3]
References:
[1] A. Kreienbrink, M. B. Sárosi, E. G. Rys and E. Hey-Hawkins, Angew. Chem. Int. Ed. 2011, 50, 4701–4703. P. Coburger, R. Aures, P. Schulz and E. Hey-Hawkins, ChemPlusChem 2018, 83, 1057–1064. P. Coburger, H. Grützmacher and E. Hey-Hawkins, Chem. Commun. 2019, 55, 3187–3190. P. Coburger, P. Bielytskyi, D. Williamson, E. Rys, A. Kreienbrink, P. Lönnecke, J. Matysik and E. Hey-Hawkins, Chem. Eur. J. 2019, 25, 11456–11465.
[2] J. Schulz, M. B. Sárosi, E. Hey-Hawkins, Chem. Eur. J. 2022, e202200531. J. Schulz, R. Clauss, A. Kazimir, S. Holzknecht, E. Hey-Hawkins, Angew. Chem. Int. Ed. 2023, 62, e202218648.
[3] P. Coburger, S. Demeshko, C. Rödl, E. Hey-Hawkins and R. Wolf, Angew. Chem. Int. Ed., 2017, 56, 15871–15875. P. Coburger, J. Schulz, J. Klose, B. Schwarze, M. B. Sárosi and E. Hey-Hawkins, Inorg. Chem. 2017, 56, 292–304. S. Bauer, I. Maulana, P. Coburger, S. Tschirschwitz, P. Lönnecke, M. B. Sárosi, R. Frank and E. Hey-Hawkins, ChemistrySelect 2017, 2, 7407–7416J. Schulz, A. Kreienbrink, P. Coburger, B. Schwarze, T. Grell, P. Lönnecke and E. Hey-Hawkins, Chem. Eur. J. 2018, 24, 6208–6216. P. Coburger, G. Kahraman, A. Straube, E. Hey-Hawkins, Dalton Trans. 2019, 48, 9625–9630. T. M. Maier, P. Coburger, N. P. van Leest, E. Hey-Hawkins and R. Wolf, Dalton Trans. 2019, 48, 15772–15777. P. Coburger, J. Leitl, D. J. Scott, G. Hierlmeier, I. Shenderovich, E. Hey-Hawkins and R. Wolf, Chem. Sci. 2021, 12, 11225-11235.
