
- This event has passed.
Seminar Dr Géraldine Masson

Dr Géraldine Masson
Institut de Chimie des Substances Naturelles (ICSN), CNRS UPR 2301, Université Paris-Saclay, 1 avenue de la Terrasse, 91198 Gif-sur-Yvette Cedex, France
Chiral Phosphoric Acid-Catalyzed Regio- and Stereocontrolled Diene and Triene Transformations for Complex Molecular Architectures
Our Conjugated trienes are versatile building blocks, yet the presence of three reactive double bonds that can be individually functionalized makes them a unique platform for constructing complex molecules, while also posing significant challenges for regio- and stereocontrolled functionalization. Unlocking their full potential requires precise catalytic strategies.[1]
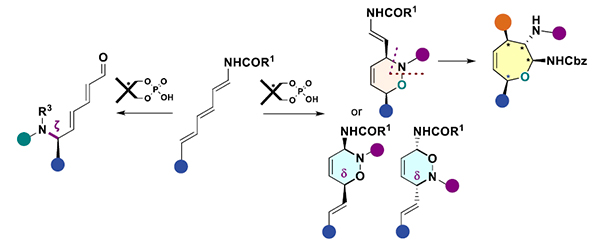
To address this, we report chiral phosphoric acid (CPA)-catalyzed transformations of NH-trienecarbamates. A regio-divergent Nitroso–Diels–Alder reaction of NH-triene-carbamates provides three of four possible cis-3,6 dihydro-2H-1,2-oxazine regioisomers with high diastereo- and enantioselectivity.[2] Interestingly, during this study we discovered an unexpected skeletal editing of one regioisomer: a diastereoselective ring expansion furnishes tetrasubstituted Δ³-oxepenes under mild conditions. [3]
Exploiting the reactivity of triene-carbamates, we also developed a remote ζ-amination of trienecarbamates, which provides densely functionalized dienals in up to 95% ee through dual hydrogenbond activation, illustrating CPA catalysis’ ability to orchestrate distal functionalization.[4]
Finally, we turned our attention to ortho-phenolic 1,3-dienes as versatile intermediates for building complex heterocycles. Through CPA-catalyzed spirocyclization of 3-indolylmethanols, we were able to achieve scaffold-divergent and enantioselective access to tetrahydrochromeno[2,3-b]indoles and tetrahydrocyclohepta[b]indoles, each bearing up to five contiguous stereocenters.[5].
Références
[1] Selected examples: (a) Nguyen, V. T.; Dang, H. T.; Pham, H. H.; Nguyen, V. T.; Flores-Hansen, C.; Arman, H. D.; Larionov, O. V. J. Am. Chem. Soc. 2018, 140, 8434. (b) Xue, Z.-J.; Li, M.-Y.; Zhu, B.-B.; He, Z.-T.; Feng, C.-G.; Lin, G.-Q.S Chem. Commun. 2020, 56, 14420D. Bouchet, T. Varlet, G. Masson, Acc. Chem. Res. 2022, 55, 3265.
[2] Naulin, E.; Lombard, M.; Gandon, V.; Retailleau, P.; Van Elslande, E.; Neuville, L.; Masson, G. J. Am. Chem. Soc. 2023, 145, 26504.
[3,4,5] In preparation.
