LCC
Cocktail catalyst
The aim is to develop supported catalysts by integrating ultra-rational use of the active phase (often consisting of a critical metal) but also of the support, which can also contribute to catalysis, in order to achieve cooperative catalysis from multifunctional catalysts.
This original concept, combining the activities of several metallic species (nanoparticles, isolated atoms) and the support (via hydrogen spillover), has been exploited to significantly improve the performance of palladium catalysts for hydrogenation reactions and nickel catalysts for CO₂ reduction.

Fig. Cocktail catalyst (credit Philippe Serp Toulouse).
References
Synergy between supported metal single atoms and nanoparticles and their relevance in catalysis
Serp P.
ChemCatChem 2023, 15(15), e202300545/1-12.
https://doi.org/10.1002/cctc.202300545
https://hal.science/hal-04174631
“Cocktail”-type catalysis on bimetallic systems for cinnamaldehyde selective hydrogenation: Role of isolated single atoms, nanoparticles and single atom alloys
Audevard J., Navarro-Ruiz J., Bernardin V., Philippe R., Corrias A., Tison Y., Favre-Réguillon A., Del Rosal I., Gerber I. C., Serp P.
Journal of Catalysis 2023, 425, 245-259.
https://doi.org/10.1016/j.jcat.2023.06.023
https://hal.science/hal-04139552
Effects of Pd and Co intimacy in Pd-modified Co/TiO2 catalysts for direct CO2 hydrogenation to fuels: the closer not the better
Scarfiello C., Durupt A., Tison Y., Pham Minh D., Soulantica K., Serp P.
Catalysis Science & Technology 2024, 14(10), 2896-2907.
https://doi.org/10.1039/d4cy00324a
https://hal.science/hal-04552701
Acetophenone hydrogenation and consecutive hydrogenolysis with Pd/CNT catalysts: Highlighting the synergy between single atoms and nanoparticles by kinetic modeling
Bernardin V., Vanoye L., Rivera-Cárcamo C., Serp P., Favre-Réguillon A., Philippe R.
Catalysis Today 2023, 422, 114196/1-9.
https://doi.org/10.1016/j.cattod.2023.114196
https://hal.science/hal-04107663
Adjustment of the single atom/nanoparticle ratio in Pd/CNT catalysts for phenylacetylene selective hydrogenation
Audevard J., Navarro-Ruiz J., Bernardin V., Tison Y., Corrias A., Del Rosal I., Favre-Réguillon A., Philippe R., Gerber I. C., Serp P.
ChemCatChem 2023, e202300036/1-18.
https://doi.org/10.1002/cctc.202300036
https://hal.science/hal-04102599
Mechanism of hydrogen spillover on metal-doped carbon materials: surface carboxylic groups are key
Navarro-Ruiz J., Audevard J., Vidal M., Campos C. H., Del Rosal I., Serp P., Gerber I. C.
ACS Catalysis 2024, 14(9), 7111-7126.
https://doi.org/10.1021/acscatal.4c00293
https://hal.science/hal-04587084
Probing basal and prismatic planes of graphitic materials for metal single atom and subnanometer cluster stabilization
Vidal M., Pandey J., Navarro-Ruiz J., Langlois J., Tison Y., Yoshii T., Wakabayashi K., Nishihara H., Frenkel A. I., Stavitski E., Urrutigoïty M., Campos C. H., Godard C., Placke T., del Rosal I., Gerber I. C., Petkov V., Serp P.
Chemistry – A European Journal 2024, 30(50), e202400669/1-18.
https://doi.org/10.1002/chem.202400669
https://hal.science/hal-04677240
Deactivation of Pd/C catalysts by irreversible loss of hydrogen spillover ability of the carbon support
Vanoye L., Guicheret B., Rivera-Cárcamo C., Audevard J., Navarro-Ruiz J., del Rosal I., Gerber I. C., Campos C. H., Machado B., Volkman J., Philippe R., Serp P., Favre-Réguillon A.
Journal of Catalysis 2023, 424, 173-188.
https://doi.org/10.1016/j.jcat.2023.05.006
https://hal.science/hal-04113228
Preparation of self-assembled supported catalysts.
The use of self-assembly reactions is a promising avenue for the development of original nano-architectures for catalytic applications.
Inspired by metal-organic frameworks, we have developed an original synthesis route that allows us to obtain assemblies of metal NPs (as well as isolated metal atoms) covalently bound by organic ligands. To date, several metals (Ru, Co, Rh, Au) and three organic motifs have been studied: C60, triphenylene- and adamantane-based ligands.
The ability to control the dimensionality of the assembly is one of the major challenges in the design and understanding of these advanced materials, which have proven to be highly active and selective in fine chemical reactions.
References
Covalent assemblies of metal nanoparticles – strategies for synthesis and catalytic applications
Y. Min, M. R. Axet, P. Serp
Dans “Recent Advances in Nanoparticle Catalysis“, edited by Nicholas Turner, Carmen Claver and Piet van Leeuwen, Springer, 2020, pp 129-197. DOI
3D Ruthenium Nanoparticle Covalent Assemblies from Polymantane Ligands for Confined Catalysis
Y. Min, H. Nasrallah, D. Poinsot, P. Lecante, Y. Tison, H. Martinez, P. Roblin, A. Falqui, R. Poteau, I. del Rosal, I. C. Gerber, J.-C. Hierso, M. R. Axet, P. Serp
Chem. Mater., 2020, 32, 2365-2378. DOI
Nanocatalysts for high selectivity enyne cyclization: oxidative surface reorganization of gold sub-2-nm nanoparticle networks
H. Nasrallah, Y. Min, D. Poinsot, T.-A. Nguyen, J. Roger, O. Heintz, F. Jolibois, R. Poteau, I. C. Gerber, Y. Coppel, M. L. Kahn, M. Rosa Axet, P. Serp, J.-C. Hierso
J. Am. Chem. Soc. Gold, 2021, 1, 187-200. DOI
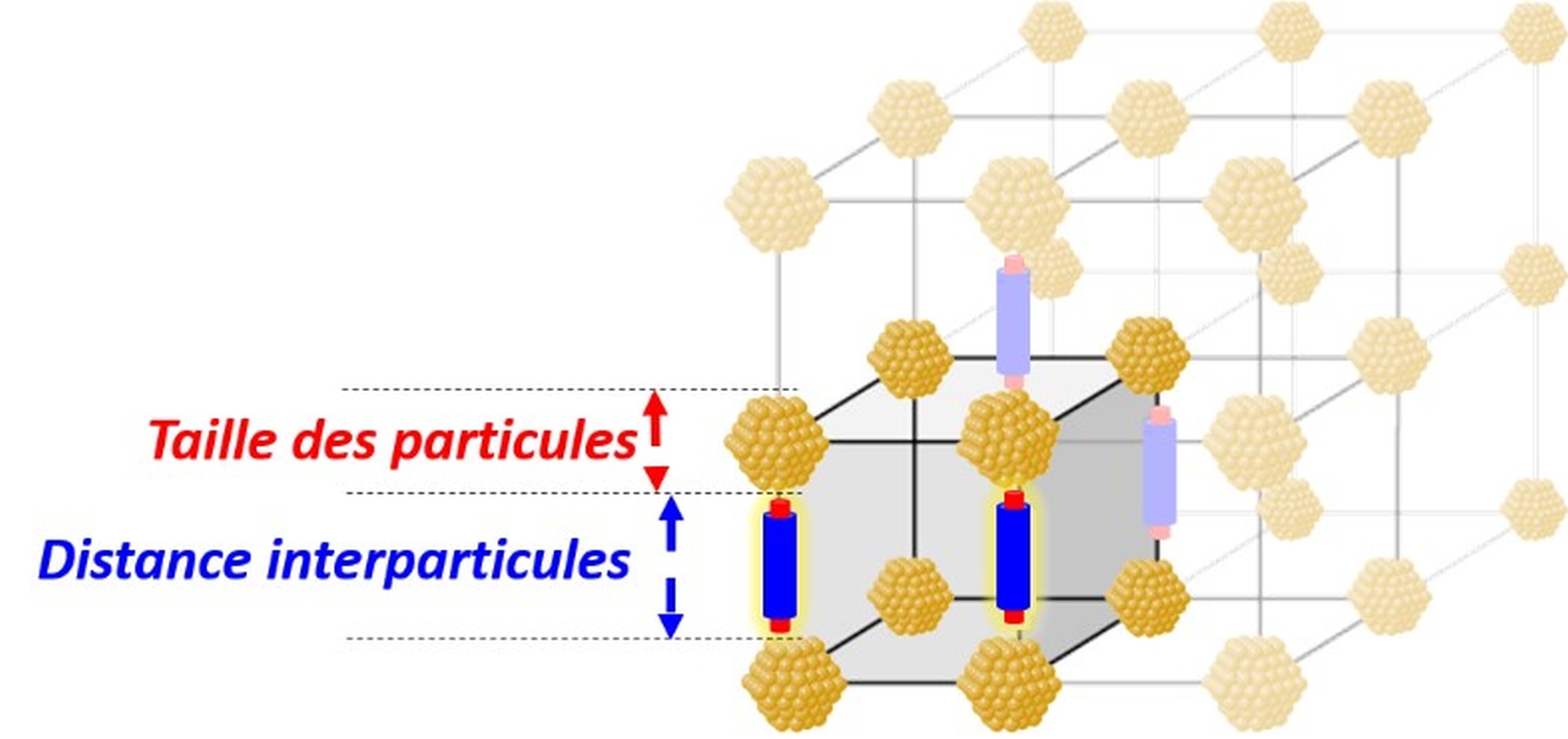
Fig. : Covalent network of metallic nanoparticles (credit P. Serp).
Supported catalysis in a confined environment
Confinement effects in heterogeneous catalysis have been the subject of particular attention because they can affect catalytic activity, selectivity and stability.
Although most studies focus on oxide-type materials, particularly zeolites, carbonaceous materials, especially carbon nanotubes, also allow such effects to occur.
Strategies are therefore being studied to selectively confine the active phase in order to highlight confinement effects in various reactions (hydrogenation, Fischer-Tropsch synthesis).
Reference
Beyond confinement effects in Fischer-Tropsch Co/CNT catalysts
A. C. Ghogia, B. F. Machado, S. Cayez, A. Nzihou, P. Serp, K. Soulantica, D. Pham Minh
J. Catal., 2021, 397, 156-171. DOI
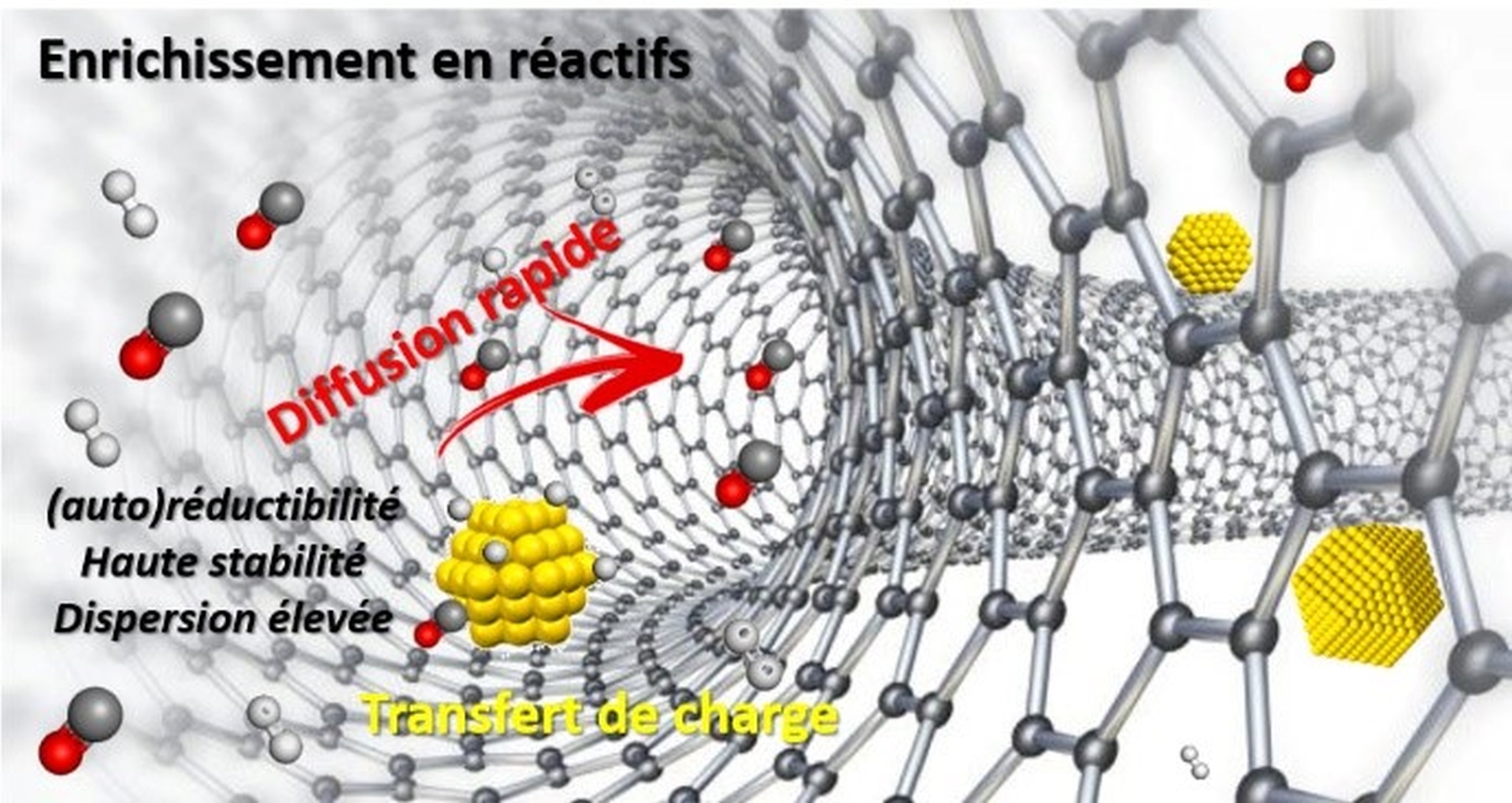
Fig. Confinement effects in supported catalysis (credit: P. Serp).
Supported catalysis for CO2 hydrogenation
This activity focuses on the hydrogenation reaction of CO2, with the objectives of controlling the selectivity of the reaction (CH4, CO, hydrocarbons) and lowering reaction temperatures.
CO2 methanation reaction. Our activities focus on developing catalysts capable of producing methane through the Sabatier reaction at low temperatures. Our efforts have concentrated on optimising a TiO2 support, both by adjusting the nature of the phases present (rutile and anatase) and by modifying the surface.
Direct Fischer-Tropsch reaction from CO2. The hydrogenation of CO2 via the Fischer-Tropsch synthesis process (CO2-FTS) produces ultra-clean synthetic fuel. This reaction is generally carried out in two stages (indirect route), combining the production of synthesis gas by reverse reaction of the gas with water in a first reactor, and a CO-FTS process in a second reactor. The direct pathway allows both reactions to be carried out at a lower temperature and in a single reactor if cobalt is used. It is this pathway, which requires the use of multifunctional catalysts, that is the subject of our research.
References
Origin of the synergetic effect between TiO2 crystalline phases in the Ni/TiO2 catalyzed CO2 methanation reaction
Messou, M. Borges Ordoño, A. Urakawa, F. Meunier, B. F. Machado, V. Collière, R. Philippe, V. Bernardin, P. Serp, C. Le Berre
J. Catal., 2021, 398, 14-28.
Tuning CO2 hydrogenation selectivity on Ni/TiO2 catalysts via sulfur addition
Le Berre, A. Falqui, A. Casu, T. T. Debela, M. Barreau, C. H. Hendon, P. Serp
Catal. Sci. Technol., 2022, 12, 6856-6864.
Low temperature Sabatier CO2 methanation
Molinet Chinaglia, S. Shafiq, P. Serp
ChemCatChem, 2024, 16, e202401213.
Modified Co/TiO2 catalysts for CO2 hydrogenation to fuels
Scarfiello, K. Soulantica, S. Cayez, A. Durupt, G. Viau, N. Le Breton, A. K. Boudalis, F. Meunier, G. Clet, M. Barreau, D. Salusso, S. Zafeiratos, D. Pham Minh, P. Serp
J. Catal., 2023, 428, 115202.
Effects of Pd and Co intimacy in Pd-modified Co/TiO2 catalysts for direct CO2 hydrogenation to fuels
Scarfiello, A. Durupt, Y. Tison, D. Pham Minh, K. Soulantica, P. Serp
Catal. Sci. Technol., 2024, 14, 2896-2907.
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




