LCC
Anisotropic magnetic materials
Magnetic anisotropy is at the origin of the magnetic properties (blocking temperature, coercive field) of molecular nano-magnets; improvement of their performance requires to increase anisotropy of the system.
Our approach is based on the control of magnetic anisotropy through rational chemical design.
It is mainly based on the use of transition or rare earth ions in pentagonal bipyramid coordination. Such complexes present magnetic anisotropy that is perfectly controlled [1] and can be used as building blocks for polynuclear networks.[2-4]
Spatial organization of local magnetic anisotropies is another parameter that we seek to control through the design of molecular components (Illustration).
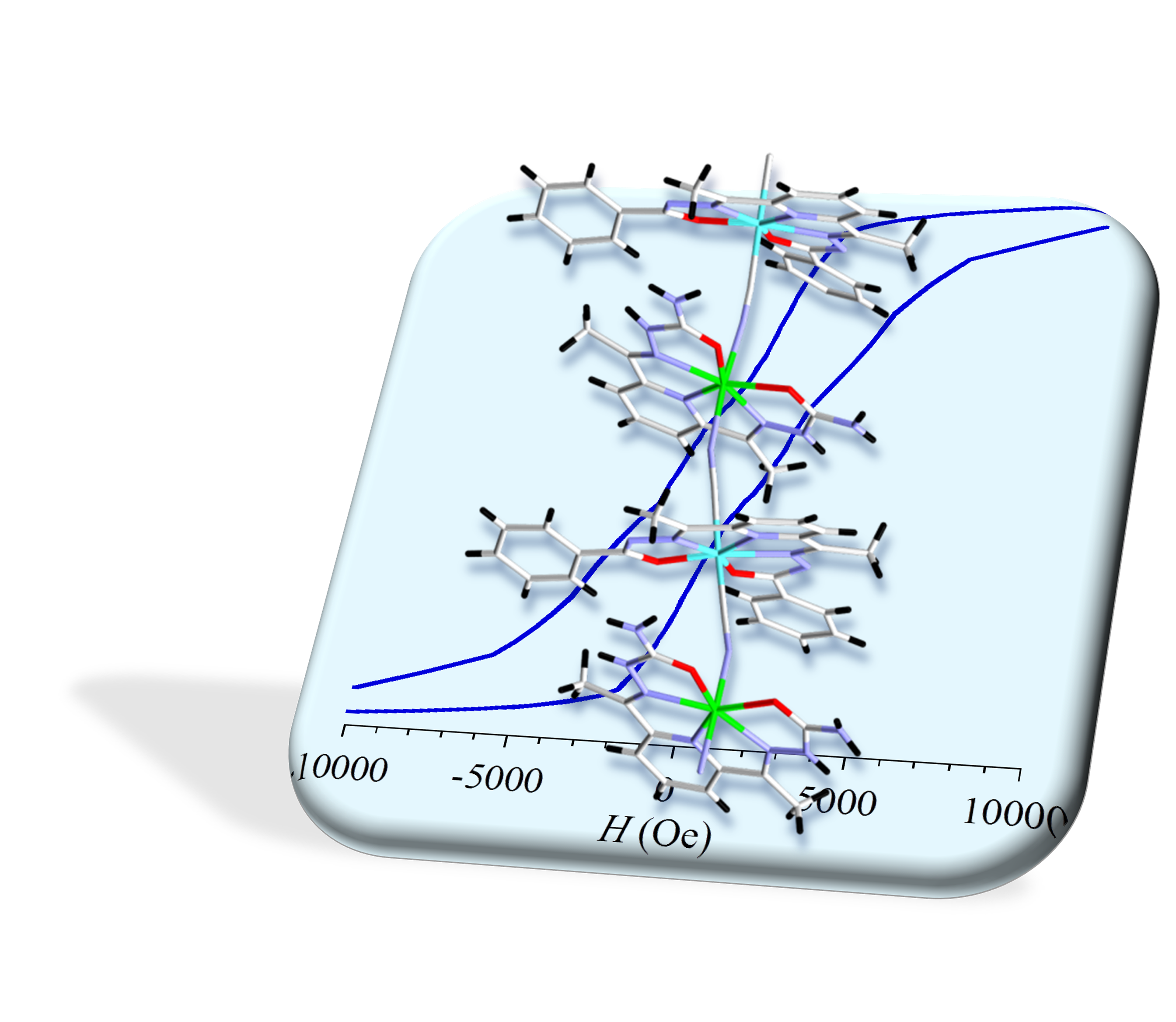
Illustration: Fe(II)–Cr(III)1D network obtained from the association of bipyramide pentagonale complexes through cyanide bridges. Quasi-alignment of individual magnetic anisotropies for each Fe ions gives rise to SCM characterized by an aimantation hysteresis below 5 K.[4]
Recent works:
[1] Magnetic anisotropy of transition metal and lanthanide ions in pentagonal bipyramidal geometry.
J.-P. Sutter, V. Béreau, V. Jubault, K. Bretosh, C. Pichon, C. Duhayon.
Chem. Soc. Rev., 2022, 51, 3280.
https://doi.org/10.1039/D2CS00028H
[2] Trinuclear cyano-bridged [Cr2Fe] complexes: to be or not to be a SMM, a matter of straightness.
C. Pichon, N. Suaud, V. Jubault, C. Duhayon, N. Guihéry, J.-P. Sutter.
Chem. Eur. J. 2021, 27, 15484.
http://dx.doi.org/10.1002/chem.202102571
[3] Ferromagnetic Ni(II)-Cr(III) Single-chain magnet based on pentagonal bipyramid building units.
K. Bretosh, V. Béreau, C. Duhayon, C. Pichon, J.-P. Sutter.
Inorg. Chem. Front 2020, 7, 1503-1511.
http://dx.doi.org/10.1039/C9QI01489F
[4] Cyano-Bridged Fe(II)–Cr(III) Single-Chain Magnet Based on Pentagonal Bipyramid Units: On the Added Value of Aligned Axial Anisotropy.
C. Pichon, N. Suaud, C. Duhayon, N. Guihéry, J.-P. Sutter.
J. Am. Chem. Soc., 2018, 140, 7698-7704.
https://doi.org/10.1021/jacs.8b03891
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




