
- This event has passed.
Seminar Pr Albrecht BERKESSEL

Pr Albrecht BERKESSEL
University of Cologne, Department of Chemistry (Organic Chemistry),
Greinstraße 4, 50939 Cologne, Germany
E-mail: berkessel@uni-koeln.de
Asymmetric Epoxidation and Hydroxylation with Titanium Salalen Catalysts and Hydrogen Peroxide
Titanium salalen complexes have solved a long-standing problem in homogeneous epoxidation catalysis: the enantioselective catalytic epoxidation of terminal (and other unactivated) olefins with hydrogen peroxide. Our most recent catalyst generation based on cis-DACH derived salalen-ligands (such as 1) combines high efficiency at low catalyst loading (typically ca. 1 mol %) with high enantioselectivity (typically > 95 % ee)[1,2]. The inherent selectivity of the catalyst allows the preferential epoxidation of terminal o
lefins in the presence of C=C double bonds with a higher degree of substitution. The use of aqueous hydrogen peroxide as a safe and environmentally friendly oxidant further adds to the utility of the method.
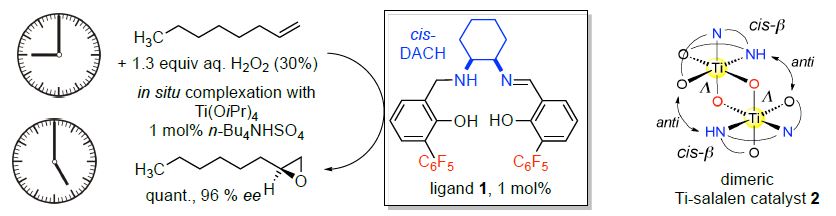
For terminal allylic alcohols (and ethers) as substrates, we could recently show that our catalyst system affords the epoxy alcohol products with virtually perfect syn-selectivity (d.r. ≥ 99:1) – it is thus complementary to the Sharpless-epoxidation which is anti-selective.[3]
The mechanistic analysis of the catalytic epoxidation process revealed the crucial role of the Tidimers 2 for H2O2 activation, together with the pentafluorophenyl moieties present in the ligand.[4] With the Ti-salalen catalyst 2 as reference compound, a central role for Ti-dimers in H2O2-activation was unveiled also for the industrial heterogeneous titanium silicalite catalyst TS-1, applied for the production of propylene oxide on 105 t/a scale.[5] Additionally, our mechanism-based ligand design recently provided a new homogeneous Ti-salalen catalyst which even hydroxylates benzylic C-H bonds, with up to 98 % ee.[6]
References
[1] For pioneering work on Ti-salalens, see: Matsumoto, K.; Sawada, Y.; Saito, B.; Sakai, K.; Katsuki, T. Angew. Chem. Int. Ed. 2005, 44, 4935-4939.
[2] (a) Berkessel, A.; Gunther, T.; Wang, Q.; Neudorfl, J.-M. Angew. Chem. Int. Ed. 2013, 52, 8467−8471. (b) Wang, Q.; Neudorfl, J.-M.; Berkessel, A. Chem. Eur. J. 2015, 21, 247-254. (c) Lansing, M.; Engler, H.; Leuther, T. M.; Neudorfl, J.-M.; Berkessel, A. ChemCatChem 2016, 68 3706−3709. (d) Berkessel, A. Aldrichimica Acta 2019, 52, 23-31.
[3] Severin, F., Fusi, G. M., Wartmann, C., Neudorfl, J.-M., Berkessel, A. Angew. Chem. Int. Ed. 2022, 61, e202201790.
[4] Engler, H.; Lansing, M.; Gordon, C. P.; Neudorfl, J.-M.; Schafer, M.; Schlorer, N. E.; Coperet, C.; Berkessel, A. ACS Catal. 2021, 11, 3206-3217.
[5] Gordon, C. P.; Engler, H.; Tragl, A. S.; Plodinec, M.; Lunkenbein, T.; Berkessel, A.; Teles, J. H.; Parvulescu, A.-N.; Coperet, C. Nature 2020, 586, 708-713.
[6] Wartmann, C., Nandi, S., Neudorfl, J.-M., Berkessel, A. Angew. Chem. Int. Ed. 2023, 62, e2023065.
