
- This event has passed.
Seminar Pr Evamarie Hay-Hawkins

Pr Evamarie Hey-Hawkins
Institute of Bioanalytical Chemistry, Leipzig University, Leipzig, Germany.
Faculty of Chemistry and Chemical Engineering, Department of Chemistry, Babes-Bolyai University,
Cluj-Napoca, Romania
Carboranes and Metallacarboranes as Building Blocks for the Design of Novel Anti-Tumour Agents
While the chemistry of carboranes has been studied extensively since the 1960ies, recent developments focus mainly on design and principles for application. Isosteric replacement strategies are applied linking the concepts of phenyl or cyclopentadienyl rings to carborane clusters (closo-C2B10H12 or nido-[7,8- C2B9H11]2−) in drug design. Carborane clusters offer unique features, such as hydrophobicity, larger steric demand, special electronic structure, or site-specific derivatisation patter, which can be superior to those of their organic counterparts in view of a specific application.[1] Thus, implementation of the carboranyl moiety as a phenyl mimetic in biologically active molecules can result in compounds with improved biological stability and activity in comparison to their generic paradigms.[2]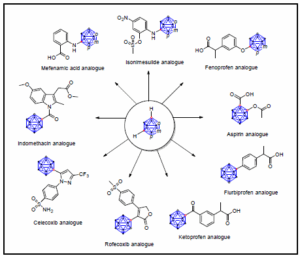
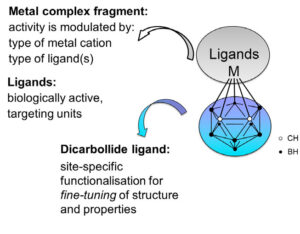
Another aspect includes applications of metallacarboranes, [3-M-1,2-C2B9H11], in biomedical chemistry.[3] This approach allows a symbiosis of the carborane features, the versatility of metals in biological systems and specific ligands, which can be selected according to their targets. In aqueous solutions, metallacarboranes spontaneously form self-assemblies in the nanometre range; their size can be efficiently controlled by formulation with bovine serum albumin (BSA).[4] These self-assembled nanoparticles might provide a selective drug delivery system, via exploitation of the well-known “enhanced permeability and retention” (EPR) effect.
The design and in vitro biological evaluation of specific carborane and metallacarborane derivatives[5] conjugated to selective vector system for targeted tumour therapy will be presented.
Références
[1] Stockmann, P. et al. Chem. Soc. Rev. 2019, 48, 3497.
[2] Selected examples from our group: Buzharevski, A. et al. ChemMedChem 2019, 14, 315; Kuhnert, R. et al. ChemMedChem 2019, 14, 255; Selg, C. et al. Chem. Sci. 2021, 12, 11873; Kuhnert, R. et al. ChemMedChem 2022, 17, e202100588n; Di Battista, V.; Hey-Hawkins, E. J. Pharm. Sci. 2022, 11, 1262; Braun. S. et al. ChemMedChem 2023, 18, e202300206; Ueberham, L. et al. J. Med. Chem. 2023, 66, 5242; Selg, C. et al. ChemPlusChem 2024, 89, e202300759; Stockmann, P. et al. ChemMedChem 2024, 19, e202300506; Selg, C. et al. Sci. Rep. 2024, 14, 30488.
[3] Hey-Hawkins, E.; Vinas, C. (eds.), Boron-based Compounds: Potential and Emerging Applications in Medicine, Wiley-VCH, 2018, ISBN 9781119275558.
[4] Selected examples from our group: Gozzi, M. et al. ChemMedChem 2019, 14, 2061. Schwarze, B. et al. ChemMedChem 2019, 14, 2075. Drača, D. et al. Molecules 2021, 26, 3801; Gozzi, M. et al. ChemMedChem 2021, 16, 1533; Kazimir, A. et al. Pharmaceutics 2023, 15, 682; Kazimir, A. et al. ChemMedChem 2024, 19, e202400006.
[5] Schwarze, B.; Gozzi, M. et al. J. Nanopart. Res. 2020, 22, 24.
