LCC
In situ study of the evolution of NiFe nanocatalysts in reductive and oxidative environments upon thermal treatments
François Robert, Pierre Lecante, Jean-Sébastien Girardon, Robert Wojcieszak, Éric Marceau, Valérie Briois, Catherine Amiens and Karine Philippot
Faraday Discuss., 2023,242, 353-373
https://doi.org/10.1039/D2FD00095D
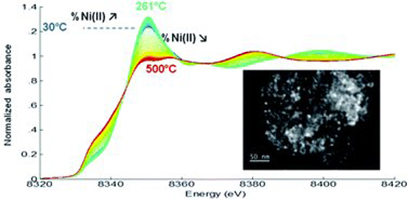
The conversion of biomass as a sustainable path to access valuable chemicals and fuels is very attractive for the chemical industry, but catalytic conversions still often rely on the use of noble metals. Sustainability constraints require developing alternative catalysts from abundant and low-cost metals. In this context, NiFe nanoparticles are interesting candidates. In their reduced and supported form, they have been reported to be more active and selective than monometallic Ni in the hydrogenation of the polar functions of organic molecules, and the two metals are very abundant. However, unlike noble metals, Ni and Fe are easily oxidized in ambient conditions, and understanding their transformation in both oxidative and reductive atmospheres is an important though seldom investigated issue to be addressed before their application in catalysis. Three types of NiFe nanoparticles were prepared by an organometallic approach to ensure the formation of ultrasmall nanoparticles (<3.5 nm) with a narrow size distribution, controlled composition and chemical order, while working in mild conditions: Ni2Fe1 and Ni1Fe1, both with a Ni rich core and Fe rich surface, and an alloy with a Ni1Fe9 composition. Supported systems were obtained by the impregnation of silica with a colloidal solution of the preformed nanoparticles. Using advanced characterization techniques, such as wide-angle X-ray scattering (WAXS) and X-ray absorption spectroscopy (XAS) in in situ conditions, this study reports on the evolution of the chemical order and of the oxidation state of the metals upon exposure to air, hydrogen, and/or increasing temperature, all factors that may affect their degree of reduction and subsequent performance in catalysis. We show that if oxidation readily occurs upon exposure to air, the metals can revert to their initial state upon heating in the presence of H2 but with a change in structure and chemical ordering.
Contributors:
- CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP 44099, F- 31077 Toulouse Cedex 4, France. E-mail: catherine.amiens@lcc-toulouse.fr
- Université de Toulouse, UPS, INPT, F-31077 Toulouse Cedex 4, France
- CNRS, CEMES (Centre d’Elaboration des Matériaux et d’Etudes Structurales), 29 Rue Jeanne Marvig, BP 4347, F-31055 Toulouse Cedex 4, France
- Univ. Lille, CNRS, Centrale Lille, Univ. Artois, UMR 8181 – UCCS – Unité de Catalyse et Chimie du Solide, F-59000 Lille, France
- Synchrotron SOLEIL, CNRS-UR1, L’Orme des Merisiers, BP48, Saint-Aubin, F-91192 Gif-sur Yvette, France
Fundings:
- Agence Nationale de la Recherche, Projet ANR “NobleFreeCat”, ANR- 17-CE07-0022-01 https://noblefreecat.univ-lille.fr/en/,
- Région Occitanie (ALDOCT 000355)
- CNRS,
- Université de Toulouse Paul-Sabatier,
- SOLEIL synchrotron (project no. 20200224).
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




