LCC
Projects in this area are based on single-site catalytic systems (supported molecular catalysts and isolated atoms) immobilised on supports, with the aim of developing processes with low environmental impact (atom economy, catalyst recycling, implementation in continuous and/or solvent-free processes).
Reference
Stabilization of metal single atoms on carbon and TiO2 supports for CO2 hydrogenation: The importance of regulating charge transfer
Rivera-Cárcamo C., Scarfiello C., García A. B., Tison Y., Martinez H., Baaziz W., Ersen O., Le Berre C., Serp P.
Advanced Materials Interfaces 2021, 8(8), 2001777/1-17.
https://doi.org/10.1002/admi.202001777, https://hal.archives-ouvertes.fr/hal-03043523
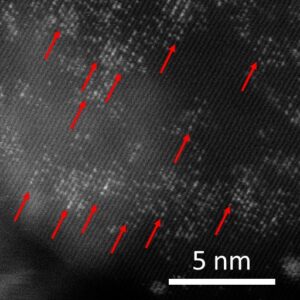
Fig. High-resolution TEM image of a single Pt atom catalyst on an MgO support. The bright spots indicated by the arrows are the isolated atoms. Credit: Bidyut B. Sarma
Supported molecular catalysts
- Immobilisation of molecular metal catalyst over supports has the advantage of leading to the development of catalytic phases that can be more easily separated from the reaction medium, or even reused, as well as the possibility of continuous use.
- In the case of carbonaceous supports, it has been shown that these can play a significant role in the catalytic reaction. We are studying the influence of various factors (such as the nature of the interaction and the distance between the catalytically active center and the support, the nature and morphology of the latter, etc.) on catalytic performance (activity, selectivity, stability). In this context, we are developing specific ligands to enable the immobilisation of different transition metal complexes from the right-hand side of the periodic table.
- The systems developed are used in particular in the asymmetric hydrogenation reaction of various substrates using gold- and rhodium-based catalysts in batch and flow processes.
- We are also interested in developing catalysts for various (co)polymerisation reactions: iron and nickel catalysts for ethylene polymerisation, palladium for CO/olefin copolymerisation, and more recently, various systems for the synthesis of polycarbonates by copolymerisation of bio-based epoxides obtained from terpenes and CO2; the latter also acting as a solvent in a process operating in a supercritical environment.
- We are also interested in developing supported catalysts for MOST systems that convert solar energy into thermal energy. Cobalt complexes have been adsorbed onto various carbonaceous supports to create recyclable catalytic systems that can be used in fixed beds for continuous flow operation.
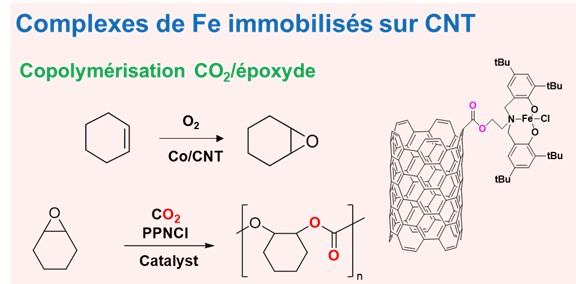
Fig. Copolymerisation of cyclohexene with CO₂ on an Fe catalyst immobilised in a CNT. (crédit J. Durand).
References
High pressure equilibrium data of CO2/cyclohexene oxide and CO2/limonene oxide systems in the context of polycarbonate synthesis using CO2 as a co-monomer, Pasini E. V., Durand J., Camy S., Fluid Phase Equilib. 2025, 595, 114406/1-11., https://www.sciencedirect.com/science/article/abs/pii/S0378381225000767
Supported Co-based catalytic systems for the epoxidation of terpenes using O2 as oxidant, Rais M., Soulié J., Tendero C., Durand J., Eur. J. Inorg. Chem. 2025, 28(5), e202400293/1-14., 10.1002/ejic.202400293 – hal-04836205 https://doi.org/10.1002/ejic.202400293
Single atom catalyst
Single-atom catalysis has become a major area of research in heterogeneous catalysis in recent years. In these systems, the absence of ensemble effects and the existence of strong electronic effects from the support induce reactivity that is very different from that of metal particles. We are developing synthesis strategies aimed at stabilizing isolated metal atoms on oxide or carbonaceous supports and studying the reactivity of these species in hydrogenation reactions.
References
Acetophenone hydrogenation and consecutive hydrogenolysis with Pd/CNT catalysts: Highlighting the synergy between single atoms and nanoparticles by kinetic modeling, Bernardin V., Vanoye L., Rivera-Cárcamo C., Serp P., Favre-Réguillon A., Philippe R., Catalysis Today 2023, 422, 114196/1-9., https://doi.org/10.1016/j.cattod.2023.114196, https://hal.science/hal-04107663
Adjustment of the single atom/nanoparticle ratio in Pd/CNT catalysts for phenylacetylene selective hydrogenation, Audevard J., Navarro-Ruiz J., Bernardin V., Tison Y., Corrias A., Del Rosal I., Favre-Réguillon A., Philippe R., Gerber I. C., Serp P., ChemCatChem 2023, 15(11), e202300036/1-18., https://doi.org/10.1002/cctc.202300036, https://hal.science/hal-04102599
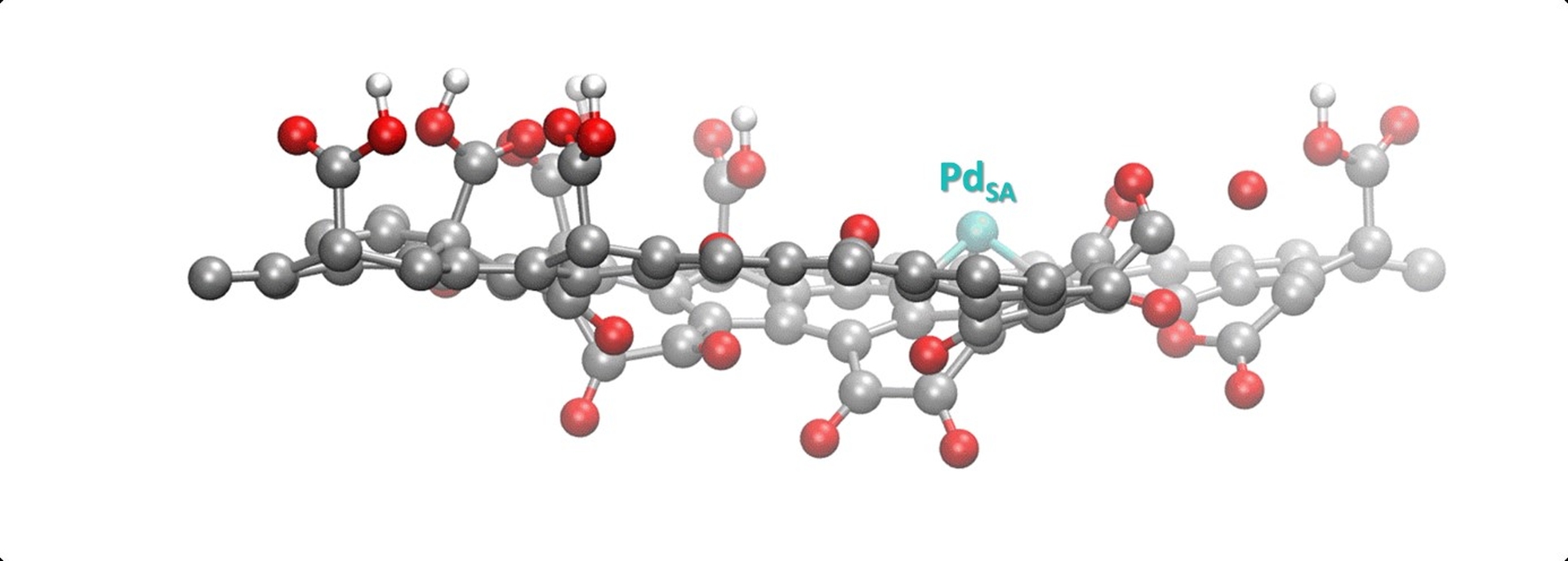
Fig. Model of a single palladium atom (PdSA) stabilised on a sheet of oxidised graphene (crédits J. Navarro Ruiz, LPCNO, Toulouse, France).
Single atom catalysts (SAC) for photocatalytic/electrocatalytic applications.
This work is being conducted in collaboration with Friedrich-Alexander University (FAU) in Erlangen-Nuremberg, Germany, and the Indian Institute of Chemical Technology (IICT) in India. As part of this work, we are studying the global and local structure of SACs using X-ray absorption spectroscopy (XAS), diffuse reflection infrared spectroscopy (DRIFTS) and high-resolution transmission electron microscopy (HR-TEM). SACs are ultimately used for the hydrogen evolution reaction under the effect of light or electricity.
References
- Single-atom catalysts on C3N4: Minimizing single atom Pt loading for maximized photocatalytic hydrogen production efficiency, Lazaar N., Wu S., Qin S., Hamrouni A., Sarma B. B., Doronkin D. E., Denisov N., Lachheb H., Schmuki P., Angew. Chem., Int. Ed. 2025, 64(6), e202416453/1-8., 10.1002/anie.202416453 – hal-04927966, https://doi.org/10.1002/anie.202416453
- Pd single atoms on g-C3N4photocatalysts: minimum loading for maximum activity, Jeyalakshmi V., Wu S., Qin S., Zhou X., Sarma B. B., Doronkin D. E., Kolařík J., Šoóš M., Schmuki P., Chem. Sci. 2025, 16(11), 4788-4795., 10.1039/D4SC08589B – hal-05064320, https://doi.org/10.1039/D4SC08589B
- Atomically dispersed Cu–Ni dual-metal sites on g-C3N4 for synergistic enhancement of photocatalytic hydrogen evolution, Islam H., Jaksani B., Iqbal A., Varangane S., Annadata H. V., Ghosh B., Sarma B. B., Thapa R., Pal U., ACS Appl. Energy Mater. 2025, 8(13), 9770-9780., 10.1021/acsaem.5c01346 – hal-05183801, https://doi.org/10.1021/acsaem.5c01346
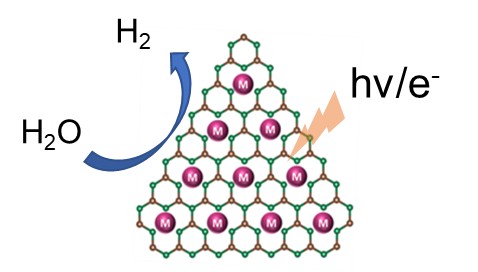
Fig. Single metal atom incorporated into C3N4, TiO2 for the hydrogen evolution reaction (crédit : Bidyut B. Sarma)
LCC CNRS
Laboratoire de chimie de coordination du CNRS
205 route de Narbonne, BP 44099
31077 Toulouse cedex 4
France




